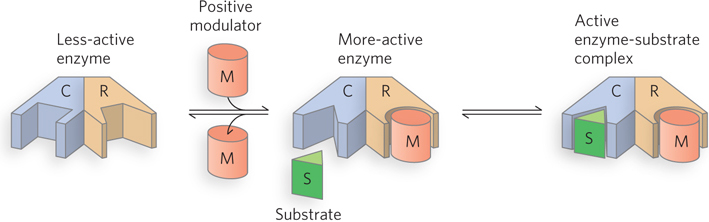
Allosteric enzyme interactions. In many allosteric enzymes, the substrate- r- s-