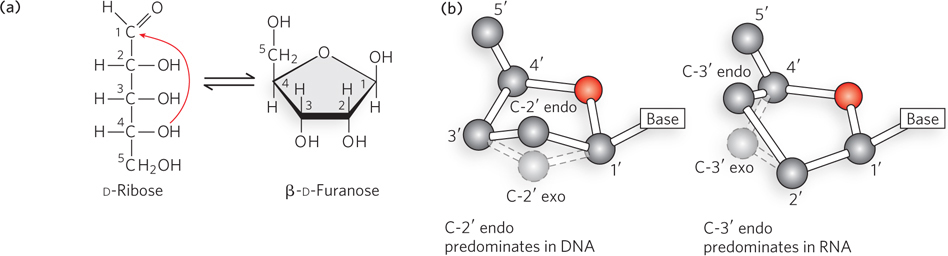
Pentose ring structures in nucleic acids. (a) The linear and closed- C- C- C- C- C- C- C- C- C- C-