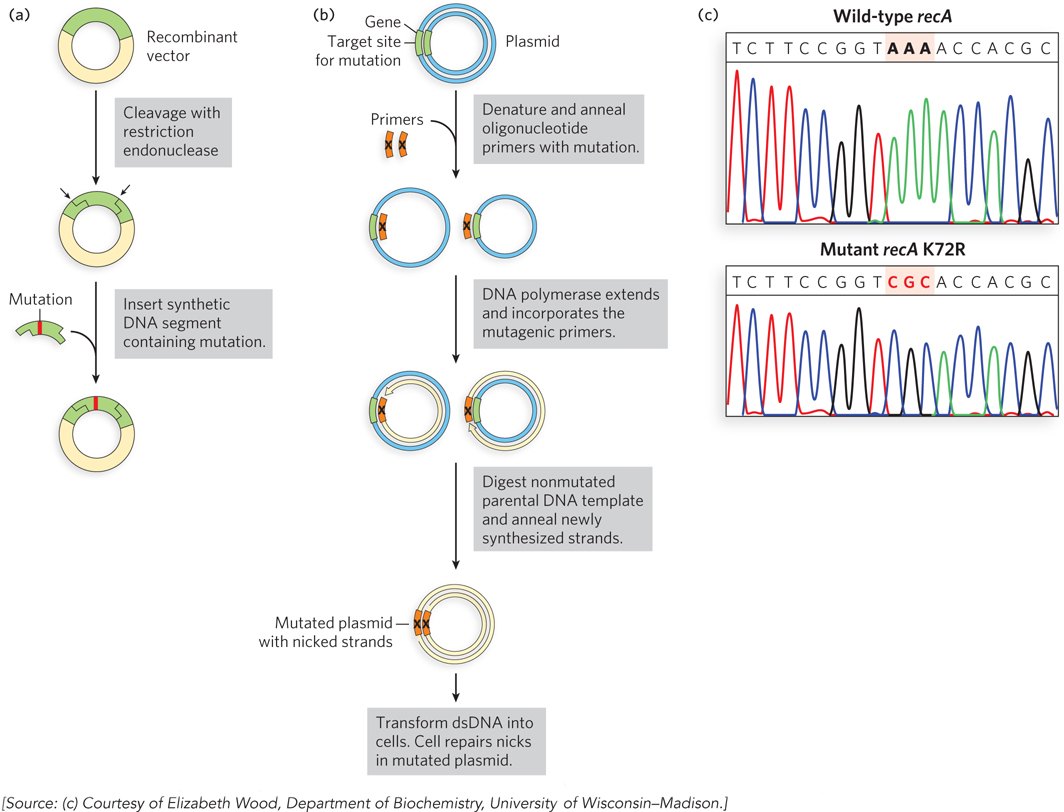
Two approaches to site- l- e- 7- d-