3.2 CHEMICAL BONDS
All molecules, whether table salt (sodium chloride) or a segment of DNA, are atomic aggregates in which the atoms are held together by attractive forces known as chemical bonds. Some chemical bonds are strong and can hold two atoms together indefinitely, but others are relatively weak and transient. In this section we discuss strong chemical bonds—
Electrons Are Shared in Covalent Bonds and Transferred in Ionic Bonds
A covalent bond is formed when two atoms share a pair of electrons between their positively charged nuclei (Figure 3-7a). Atoms joined by a covalent bond tend to share electrons such that their outer electron shells are filled. Another type of chemical bond is an ionic bond, which, in contrast to covalent bonds, involves the complete transfer of one or more electrons from one atom to another. When electrons are transferred, the atoms are converted into ions—
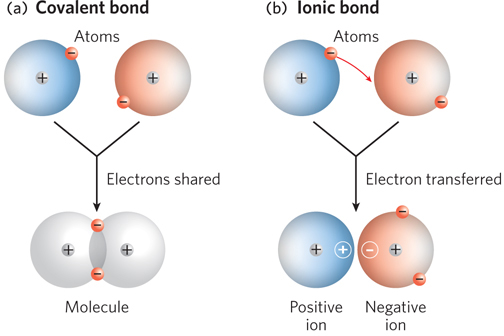

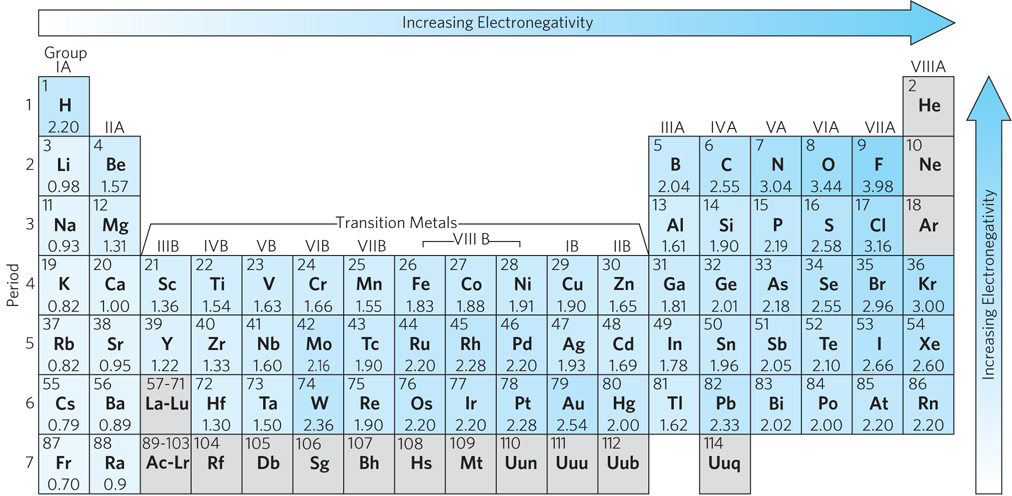
In the 1930s, Linus Pauling demonstrated that covalent bonds and ionic bonds represent opposite ends of a continuum; in reality, most chemical bonds lie somewhere in between. To determine whether two atoms in a molecule are more likely to form a covalent or an ionic bond, Pauling introduced the concept of electronegativity, the propensity of an atom within a molecule to attract electrons to itself. Unequal electron sharing reflects different affinities of the bonded atoms for electrons. Atoms with a tendency to gain electrons are referred to as electronegative atoms, those with a propensity to lose electrons as electropositive atoms. In general, as atomic radius decreases, electronegativity increases and the atom has a greater likelihood of forming an ionic rather than a covalent bond. Ionic bonds often form between a metal and a nonmetal; the metal atom donates one or more electrons to the nonmetal atom to form a salt, such as sodium chloride. When Pauling formulated the concept of electronegativity, he developed a scale of dimensionless values ranging from 0.7 to 3.98 and assigned a value to each atom (e.g., hydrogen = 2.20). With this Pauling scale, the difference in electronegativity of two atoms can be used to predict the type of bonding between them (Figure 3-8). When this difference is zero or very small, the bond is purely covalent; when the difference is greater than zero but less than 1.67, the bond is considered to be polar covalent, meaning that the electrons are shared between the atoms but biased toward one “pole” of the two-
69
Although typically weaker than covalent bonds, ionic bonds do not restrict the relative orientations of the bonded atoms; thus, they are very useful in macromolecules. For example, ionic bonds—
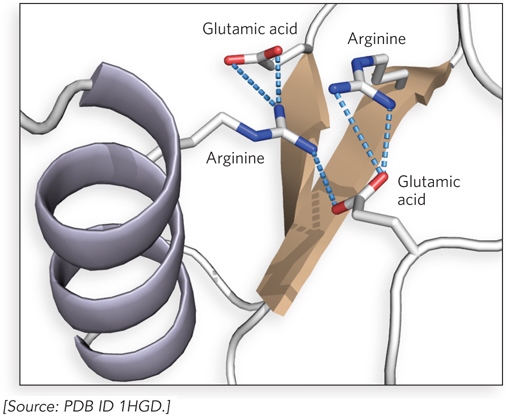
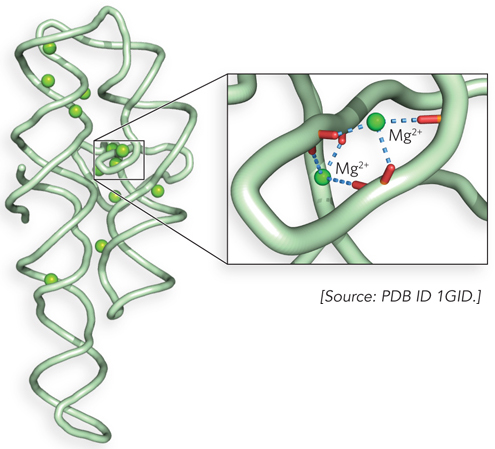
The strength of an ionic bond can vary with the salt concentration and hydrophobicity of the environment. Some ionic bonds are very strong indeed. Sodium chloride is a good example of a molecule with a single strong ionic bond. Like other metals, sodium tends to form ionic bonds because it loses an electron, producing a positive ion that is strongly attracted to the negatively charged chloride ion. However, many ionic bonds found in biological molecules are weaker than the strong ionic or covalent bonds.
70
Chemical Bonds Are Explainable in Quantum Mechanical Terms
Although the idea of shared electron pairs provides a useful qualitative description of covalent bonding, the nature of the strong and weak forces that produce chemical bonds remained unknown to chemists until the development of quantum mechanics in the 1920s. The German scientists Walter Heitler and Fritz London offered the first successful quantum mechanical explanation of molecular hydrogen in 1927, laying the foundation for predicting the structures and properties of other simple molecules. Their work was based on the valence bond model, which posits that a chemical bond forms when there is suitable overlap between the electron clouds, or atomic orbitals, of participating atoms. These atomic orbitals are known to have specific angular interrelationships, and thus the valence bond model can predict the bond angles observed in simple molecules. Today, the valence bond model has been supplemented with the molecular orbital model, in which the atomic orbitals of bonded atoms interact to form hybrid molecular orbitals. These molecular orbitals extend between the two bonding atoms.
Each element forms a characteristic number of bonds necessary to give it a complete outer shell of electrons. Because a complete outer shell, for most atoms, contains eight electrons, this is known as the octet rule. The maximum number of covalent bonds a particular atom can form is called its valence. The valence of the atoms commonly found in biological molecules dictates the shape, chemical properties, and ultimately the behavior of these molecules, even for large polymers such as nucleic acids and proteins. Hydrogen, oxygen, nitrogen, and carbon have valences of 1, 2, 3, and 4, respectively. Thus, hydrogen can form just one covalent bond, and O, N, and C can form any combination of single or multiple bonds to make up the total allowable number (Figure 3-11). A single bond between two atoms involves two electrons. Four shared electrons between two atoms produce a double bond (Figure 3-12).
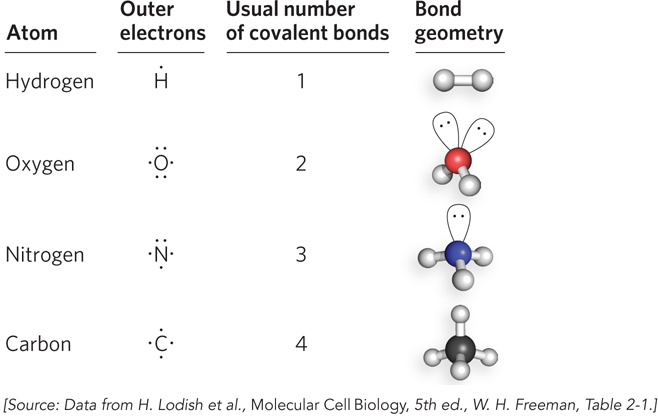
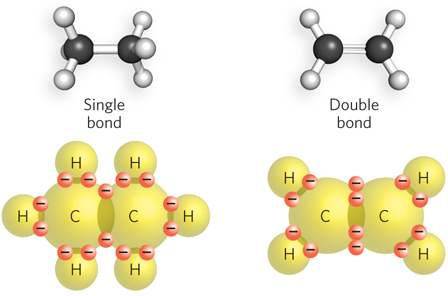
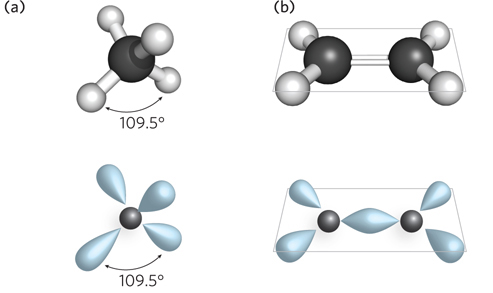
The angle between two bonds originating from a single atom is called the bond angle. The angle between two specific types of covalent bonds is always approximately the same. For example, the four single covalent bonds of a carbon atom are directed toward the corners of a tetrahedron (bond angle = 109.5°) (Figure 3-13). Covalent bonds differ in the degree of rotation they allow. Single bonds permit free rotation of the bound atoms around the bond, whereas double bonds are more rigid.
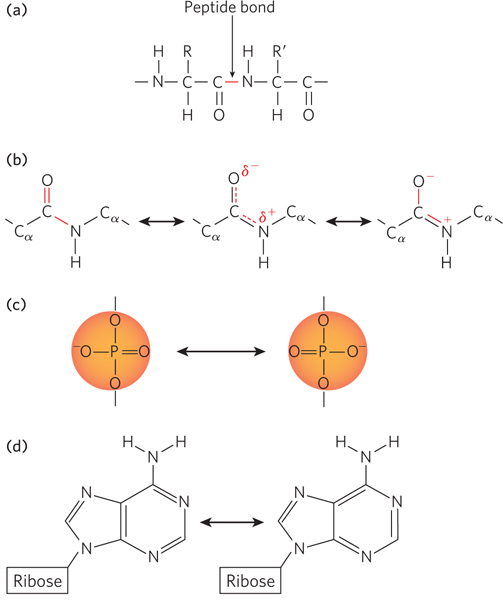
Many molecules that contain single and double bonds adjacent to each other can exist as an average of multiple structures, a phenomenon called resonance. A resonance hybrid is a molecule that exists in an average of two possible forms. A classic example of this in biology is the molecular structure around the peptide bond that links together two amino acids (Figure 3-14a). The peptide bond links the carboxyl group of one amino acid to the amino group of another. The carbonyl (C=O) and imino (C=N) bonds each have both double-
71
72
Resonance also affects the behavior of nucleic acids, in multiple ways. The phosphodiester bonds that link the individual nucleotides of DNA and RNA have a tetrahedral geometry and include two bonds to oxygen atoms that are related by resonance (Figure 3-14c). As a consequence, the negative charge on the phosphate group can shift between the two oxygen atoms that are not bonded to sugars in the backbone. Also, the bases are conjugated ring systems—
Forming and Breaking Chemical Bonds Involves Energy Transfer
For a chemical bond to form, the total energy of the system—
A calorie is the amount of energy needed to raise the temperature of 1 gram of water by 1 degree Celsius, from 14.5°C to 15.5°C. (Another unit of energy is the joule, equal to 0.239 calories, which is defined as the energy required to apply a force of 1 newton through a distance of 1 meter.) Energy changes in chemical reactions are typically expressed in kilocalories per mole (kcal/mol), because thousands of calories are involved in forming or breaking a mole of (that is, 6.02 × 1023) chemical bonds. If energy is given off when two atoms combine to form a covalent bond, then the separated atoms must have had more total energy than the molecule. The amount of energy required to break a chemical bond exactly equals the amount that was released on its formation. This equivalence follows from the first law of thermodynamics, which states that energy, except where interconvertible with mass, can be neither created nor destroyed (see Section 3.6). This, then, is what holds atoms together in covalent bonds: they cannot separate unless they are given the required amount of energy.
Bonds frequently break on heating, because heat speeds up molecular motions, leading to intermolecular collisions in which some of the kinetic energy of a moving molecule is released as it pushes apart two bonded atoms. Higher temperatures produce faster-
Electron Distribution between Bonded Atoms Determines Molecular Behavior
All chemical bonds, whether strong or weak, are the result of attractions between electrical charges. For example, the hydrogen molecule (H:H) has a symmetric distribution of electrons between its two hydrogen atoms, so both atoms are uncharged and the bond they share is purely covalent. In contrast, the polar covalent bonds of a water molecule (H:O:H) have a nonuniform distribution of charge. In water, the bonding electrons are unevenly shared due to the different electronegativity of H and O atoms (see Figure 3-8). In this case, the oxygen atom holds the bonding electrons more strongly and thus has a considerable negative charge, whereas the two hydrogen atoms together have an equal amount of positive charge (Figure 3-15a). Such a combination of separated positive and negative charges is called an electric dipole moment.
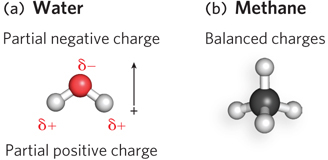
Molecules such as water that have a dipole moment are referred to as polar molecules. Nonpolar molecules are those with no effective dipole moment; an example is methane (Figure 3-15b). The large size of proteins and nucleic acids allows polar and nonpolar regions to exist within the same molecule. For example, the outer surfaces of proteins that function in the aqueous environment of the cytoplasm tend to be polar, thereby favoring interactions with polar water molecules. In contrast, proteins that function in the nonpolar environment of cellular membranes tend to have nonpolar surfaces, thus fostering contacts with the nonpolar fatty acid chains of the membrane.
73
SECTION 3.2 SUMMARY
All molecules consist of atoms linked together by strong and/or weak chemical bonds.
Covalent bonds share electrons equally between two atoms, whereas ionic bonds have electrons that are completely transferred from one atom to another, such that the charged atoms (ions) are drawn together by electrostatic forces. Electronegativity, a measure of how strongly an atom attracts electrons to itself, can be used to predict the type of bonding between two atoms.
Valence is the maximum number of covalent bonds an atom can form, and the valence of the atoms in biological molecules dictates the shape of these molecules. Carbon, with a valence of 4, forms four single bonds to neighboring atoms arranged in a tetrahedral geometry.
Resonance is an aspect of valence bond theory used to graphically represent and mathematically model molecules for which no single, conventional model can satisfactorily represent the observed molecular structure or explain its behavior. Such molecules are considered to be an intermediate or average, or resonance hybrid, of several conventional models that differ only in the placement of the valence electrons.
Single bonds, in which a pair of atoms share two electrons, give rise to variable geometries, whereas double bonds, involving four shared electrons, give rise to planar molecular geometries.
Exothermic bond formation is energetically favorable, and the total energy of the system is decreased in the process. The amount of energy released when a chemical bond breaks is the same as that required for its formation. Energy can be expressed in units of calories (cal) or kilocalories (kcal), or in joules. Molecular biologists typically describe energy changes in chemical reactions in terms of kilocalories per mole (kcal/mol).
Attractions between electrical charges in atoms lead to chemical bond formation. Two bonded atoms are uncharged when the bonding electrons are positioned equally between them. When one atom holds the bonding electrons more tightly than the other, due to differences in the atoms’ electronegativities, an unequal charge distribution results. One end of the molecule carries a net negative charge, the other a net positive charge. The molecule is said to have an electric dipole moment.
Polar molecules are those with a dipole moment, such as water. Some molecules, such as methane, lack a dipole moment and are nonpolar. The polarity of biomolecules governs their locations within cells and their interactions with other molecules.