3.5 THE ROLE OF pH AND IONIZATION
Although pH and the ionization potential of molecules may seem like esoteric topics for biology, they are central to the function of biological molecules. This is because most biological processes occur within a narrow pH range, and cells and their internal compartments carefully regulate pH to suit their needs. For example, foreign molecules that enter cells are degraded in lysosomes, organelles that maintain an interior pH of 4.5, an acidic environment that is optimal for the function of the lysosome’s digestive enzymes. The pH of blood must be maintained near neutral pH—
The Hydrogen Ion Concentration of a Solution Is Measured by pH
Many of the macromolecules and chemical reactions of central importance in molecular biology naturally occur in aqueous solutions, which are mixtures of molecules dissolved in water. Such solutions can be acidic, neutral, or basic, depending on the concentration of positively charged water molecules, called hydronium ions (H3O+). For example, a solution with [H3O+] = 4 × 10−3 mol/L is more acidic and less basic than one with [H3O+] = 5 × 10−4 mol/L. Note that we consider hydronium ions rather than hydrogen ions (H+), because any H+ ions (protons) in an aqueous environment quickly bind to water molecules to form H3O+. Because small numbers such as 4 × 10−3 or 5 × 10−4 are difficult to work with, Sören Sörenson devised another way to express [H3O+]. In 1909, he defined the quantity pH as the negative logarithm of the hydronium ion concentration:
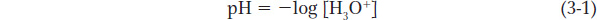
It is simple to convert [H3O+] to pH, and vice versa.
Similarly, we can define pOH as −log [OH−]. We can easily express the acidity or basicity of an aqueous solution by using pH and pOH; by common practice, however, pH is used rather than pOH. In aqueous solutions at equilibrium, the product [H3O+][OH−] = 10−14, and pH + pOH must equal 14. Thus, a solution with a pH of 4.6 has a pOH of 9.4. A solution of pH 7 is neutral, because [H3O+] = [OH−]. In a solution with a pH lower than 7, [H3O+] > [OH−] and the solution is acidic; in one with a pH greater than 7, [H3O+] < [OH−] and the solution is basic. The lower the pH, the more acidic the solution; the higher the pH, the more basic the solution. Because pH values are on a logarithmic scale, a change in pH of one unit corresponds to a tenfold change in hydronium ion concentration.
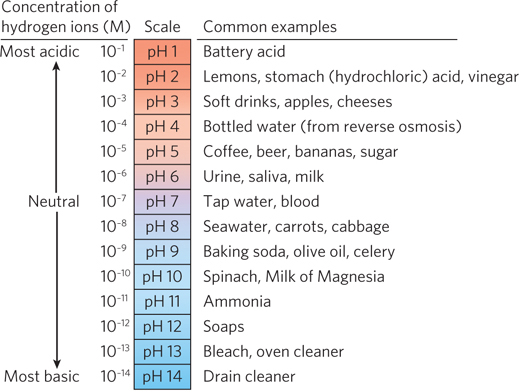
In the laboratory, the pH of an aqueous solution can be determined by using chemical compounds (pH indicators) that change color over a narrow pH range, or with an instrument called a pH meter. Many foods and other common household substances are acidic or basic aqueous solutions. Figure 3-24 lists approximate pH values of some foods and common materials, as well as some body fluids.
Buffers Prevent Dramatic Changes in pH
Most macromolecules found in biological systems have evolved to function at approximately neutral pH, because most cells and body fluids (other than stomach acid) are neither acidic nor basic. For example, the pH of normal human blood is 7.4, a value that is critical for the health of an individual. If the pH of blood were to rise to 7.8 or drop to 7.0, serious illness or death could result, because the hemoglobin protein in red blood cells would no longer be able to bind and release oxygen efficiently. The body is able to maintain the correct pH of blood, despite the constant influx of nutrients with much higher or lower pH, because blood is buffered.
82
A buffer solution, a solution with a pH that does not change very much when H3O+ or OH− ions are added, contains approximately equal amounts of a weak acid—

Buffers are present in biological fluids, and they can also be prepared in the laboratory. A typical buffer solution might consist of 0.1 mol of acetic acid (CH3COOH) and 0.1 mol of sodium acetate (CH3COONa) dissolved in 1 L of water. Not much of the acetic acid, a weak acid, loses a proton, but just about all of the sodium acetate, an ionic compound, will dissociate into the separate ions, generating the conjugate base of acetic acid. Such a solution will have equal concentrations of the weak acid acetic acid (CH3COOH) and its conjugate base, the acetate ion (CH3COO−). This solution acts as a buffer because it neutralizes any H3O+ or OH− that may be added to the solution by accepting or donating a proton, respectively—
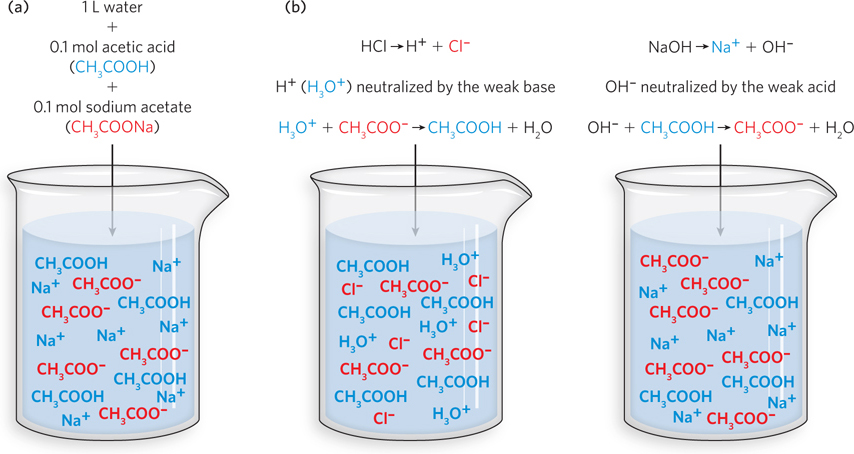
Every buffer solution has two major properties: its pH value and its buffering capacity. The buffer solution has a characteristic value called its acid dissociation constant (Ka), which equals the concentration of conjugate base multiplied by the concentration of H3O+, divided by the concentration of weak acid. We can write this as follows:
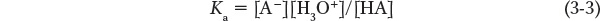
where HA is the weak acid and A− is its conjugate base. Just as we use pH to describe the H3O+ concentration on a log scale, we can define pKa = −log Ka. Each acid has a characteristic pKa value that describes the ratio of charged (A−) to neutral acid (HA) molecules at equilibrium in water. A pKa is therefore a measure of the tendency for an acid to lose its proton in aqueous solution. The lower the pKa, the stronger the acid and the stronger its tendency to give up its proton. According to Equation 3-
83
Note that a buffer solution has a limit to its ability to neutralize acid or base, beyond which its buffering action is overwhelmed. This buffering capacity depends on the total concentrations, rather than the ratio, of HA and A−. For example, consider a buffer solution Y containing 10 times more molecules of acetic acid and its conjugate base than does buffer solution Z. Both solutions have the same pH (~5), but solution Y has 10 times the buffering capacity of buffer Z because it has 10 times more molecules available to neutralize H3O+ and OH− ions.
The Henderson-Hasselbalch Equation Estimates the pH of a Buffered Solution
A defined relationship exists between the pH of a solution and the concentration of a weak acid dissolved in it. The relationship is defined by the Henderson-
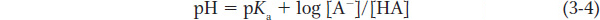
In other words, the pH of a solution containing a buffering weak acid equals the pKa of that weak acid plus the log of the ratio of the concentration of its basic and acidic forms. Using this equation, it is possible to calculate the pH of a buffered solution. Or, if the solution pH and the concentrations of the basic and acidic forms of the weak acid are known, the pKa of the weak acid can be determined.
This information can be very useful for working with biological samples, such as blood or proteins, where the pH of the solution must be carefully controlled to avoid destruction of the sample. Molecular biologists use the Henderson-
SECTION 3.5 SUMMARY
Aqueous solutions can be acidic, neutral, or basic, depending on the concentration of hydronium ions (H3O+) present.
The pH value is defined as the negative logarithm of the hydronium ion concentration:
pH = −log [H3O+]
A solution with a pH lower than 7 is acidic, and [H3O+] > [OH−]; a solution with a pH greater than 7 is basic, and [H3O+] < [OH−]. Because pH values are on a logarithmic scale, a change in pH of one unit corresponds to a tenfold change in hydronium ion concentration.
A buffer solution is a solution with a pH that does not change very much when H3O+ or OH− ions are added. It contains approximately equal amounts of a weak acid and its conjugate base.
Each acid has a characteristic pKa value, defined as pKa = −log Ka. The pKa describes the ratio of charged (A−) to neutral acid (HA) molecules at equilibrium in the solution.
The relationship between the pH of a solution and the concentration of a weak acid (HA) dissolved in it is described by the Henderson-
Hasselbalch equation: pH = pKa + log [A−]/[HA]