PROBLEMS
Question 3.1
Consider the O–
Question 3.2
Do two enantiomers of a chemical have the same density? The same melting point? If the chemical is an acid, do they have the same pKa?
Question 3.3
Which of the following statements about a catalyst is correct?
It can change the equilibrium constant of a chemical reaction.
It speeds up the rate of the forward but not the reverse reaction.
It is used up in the course of a reaction.
It lowers the activation energy for a reaction.
Question 3.4
A solution with pH 7 is 100 times more basic than a solution with a pH of what value?
Question 3.5
Amino acids are joined by peptide bonds, the formation of which is accompanied by the loss of water. Is the dipeptide alanylglycine the same as the dipeptide glycylalanine? Why or why not? (Note that peptides are always written with the amino-
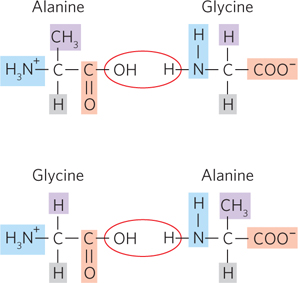
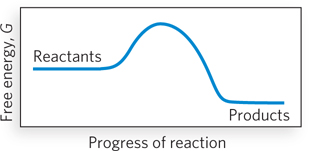
Question 3.6
For the reaction coordinate diagram shown below, the activation energy is larger when the reaction proceeds in which direction?
Question 3.7
A flask contains 10 mL of salt water. If 10 mL of distilled water is added, does the number of moles of sodium chloride increase by 50%, decrease by 50%, or remain unchanged?
Question 3.8
Which law of thermodynamics explains why living things require the input of energy to maintain their ordered structure?
Question 3.9
One of the two resonance structures for a formate ion is shown below. Which carbon–
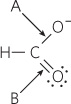
Question 3.10
The activation energy for a chemical reaction can be determined in which of the following ways?
Measuring product amounts
Measuring rates
Calculating energy of bond hydrolysis
Calculating change-
in- entropy values
Question 3.11
What is the pH of the solutions with the following hydrogen ion concentrations?
1.75 × 10−5 m
6.50 × 10−10 m
92
1.0 × 10−4 m
1.50 × 10−5 m
Question 3.12
What is the hydrogen ion concentration of the solutions with the following pH values?
3.82
6.53
11.11
Question 3.13
Calculate the pH of dilute solutions that contain the following molar ratios of acetate to acetic acid (pKa = 4.70).
2:1
1:3
5:1
1:1
1:10
Question 3.14
A buffer contains 0.01 mol of lactic acid (pKa = 3.60) and 0.05 mol of sodium lactate per liter.
Calculate the pH of the buffer.
Calculate the change in pH after 5 mL of 0.5 m HCl is added to a liter of the buffer.
Calculate the change in pH after the same quantity of this acid is added to a liter of pure water.
Question 3.15
An unknown compound is thought to have a carboxyl group with a pKa = 2.0 and a second ionizable group with a pKa between = and 8. When 75 mL of 0.1 m NaOH was added to 100 mL of a 0.1 m solution of this compound at pH 2.0, the pH increased to 6.72. Calculate the pKa of the second group.
Question 3.16
The base thymine (see Figure 3-1) contains a six-
Question 3.17
Free rotation is possible around single bonds, but not around double bonds. The repeating structure of the backbone of a polypeptide is shown below, as it is usually drawn. The bond torsion angles, describing rotation about these bonds, are labeled phi (Φ), psi (ψ), and omega (ω). In reality, significant rotation can occur about just two of these bonds. For which bond is free rotation most restricted, and why?
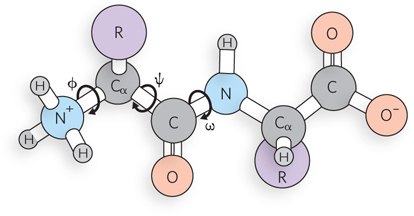
Question 3.18
The two sets of reactants shown below represent the starting points for (a) amide formation and (b) phosphoryl group transfer. In each panel, draw the curved arrows needed to indicate the first step in each reaction. Do not draw any additional intermediates or steps.
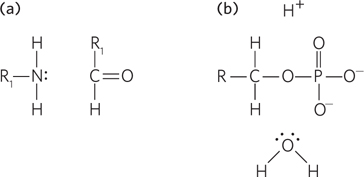
Question 3.19
The structure of one of the two stereoisomers of alanine is shown below. A pure solution of this stereoisomer is dextrorotatory. Is this molecule D-
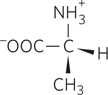
Question 3.20
A biochemist wishes to use histidine to buffer a solution at pH 6.6. Histidine has three ionizable groups, with pKa values of 1.82, 6.00, and 9.17. The biochemist has 100 mL of a 0.1 m histidine solution at pH 1.82. What volume of 1.0 m NaOH must she add to bring the pH to 6.6?