DATA ANALYSIS PROBLEM
Hershey, A.D., and M. Chase. 1952. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J. Gen. Physiol. 36:39–
Question 6.18
The stage occupied by Watson and Crick in 1953 was set by many other scientists, primarily Alfred Hershey and Martha Chase (see the How We Know section in Chapter 2). By 1952, a number of experiments had pointed to DNA as the genetic material, but much controversy remained, and protein was still regarded as a prime candidate. The Hershey and Chase experiments published in 1952 are credited with eliminating any remaining doubt in the scientific community that DNA alone was the genetic material.
The experiments made use of E. coli and one if its viruses, bacteriophage T2. When the experiments began, it was known that T2 consisted of a DNA core surrounded by a protein coat. T2 attached itself to the bacterial host and injected its core material into the host cell. New copies of T2 were made within the host, and the bacterial cell was lytically destroyed when the new T2 copies were released. Although it was clear that the T2 protein coat remained outside the bacterial cell, many workers thought that both DNA and some protein were introduced into the host when the genetic material was injected by T2, or that the attached protein shells played a role in the production of the T2 progeny. In either case, it was possible that protein was the genetic material.
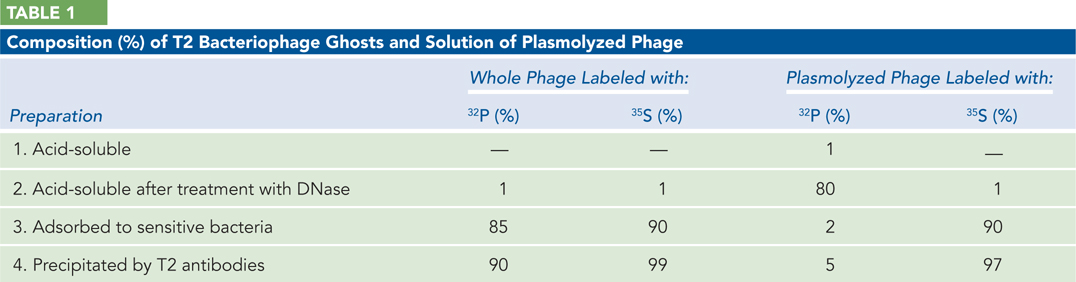
Prior to their famous blender experiment (described shortly), Hershey and Chase carried out a series of experiments to better establish what parts of the T2 bacteriophage were introduced into the bacterial cells. They knew that T2 could be inactivated by osmotic shock, leaving behind T2 “ghosts” consisting of the viral coats bereft of their internal contents (which were released into the medium). Two different batches of T2 bacteriophage were grown, one labeled with 35S and the other with 32P. Half of each preparation was subjected to osmotic shock by incubation in 3 m NaCl followed by rapid dilution into distilled water. The authors described preparations thus treated as “plasmolyzed.” Each of the four resulting preparations was further subjected to four additional treatments: (1) Addition of acid, followed by centrifugation; the supernatant was monitored for acid-
209
What macromolecules are labeled by 35S? What macromolecules are labeled by 32P?
Why didn’t the researchers use other common radioactive labels that were available at the time (14C and 3H)?
Little or no radioactivity appears in the first acid-
soluble supernatant in any of the preparations. Explain. Most of the 32P label, but little of the 35S, appears in the acid-
soluble supernatant after DNase treatment. Explain. In the samples not treated with osmotic shock, most of both labels was found adsorbed to bacteria (in the cell pellet), but only the 35S label appeared in the cell pellet in the plasmolyzed samples. Explain.
In the control samples, both labels were precipitated by the T2 antibodies, but only the 35S was precipitated in the plasmolyzed samples. Explain.
What general conclusions can you draw from these experiments?
Several additional experiments, combined with those above, established that the bulk of the phage DNA was introduced into the cells during infection and thus might contribute to the production of progeny. About 30% of the 32P label ended up in the progeny phage. But what happened to the phage protein? This consideration led to the blender experiment. Electron micrographs had previously shown that T2 attaches to the outside of bacterial cells and is attached to them by a long tail. The researchers reasoned that this “precarious attachment” could be eliminated by shearing.
Bacteria were infected with either 32P-
or 35S- labeled phage. Two different multiplicities of infection (number of phage added per cell) were used, 0.6 and 6.0. After a few minutes, the cells were centrifuged and resuspended in fresh medium. Some were subjected to a 2.5- minute treatment in a blender. Samples were then taken. For one sample, the cells were centrifuged and the supernatant measured to determine how much isotope had been removed from the cells. Another sample was left to produce phage progeny and titrated to determine what fraction of the infected cells was producing phage. The results are shown in Table 2 below. 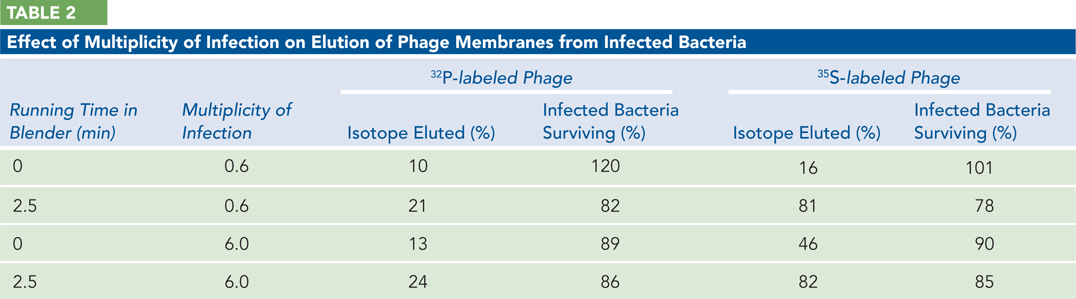
Why did the investigators centrifuge and then resuspend the phage after infection?
How much of the 35S label is stripped from the cells by the blender?
What general conclusions can you draw from this experiment?
A careful subsequent examination of the progeny phage indicated that less than 1% of the 35S label ended up in the progeny. Combining this with the other data, the researchers could make a clear case that DNA was the genetic material guiding the production of phage progeny.