PROBLEMS
Question 9.1
The diameter of a typical human cell nucleus is approximately 6 μm. Human chromosome 2 consists of approximately 243 million contiguous base pairs. Calculate the length of chromosome 2 if it were extended in a relaxed B-
Question 9.2
What is the superhelical density (σ) of a closed-
Question 9.3
The T4-
Calculate the length of the DNA (assume the molecular weight of a nucleotide pair is 650) and compare it with the length of the JS98 head.
Consult the online database Genome (www.ncbi.nlm.nih.gov/
genome). What is the exact number of base pairs in the JS98 genome?
Question 9.4
The base composition of phage M13 DNA is A, 23%; T, 36%; G, 21%; C, 20%. What does this tell you about the DNA structure of phage M13?
Question 9.5
The complete genome of the simplest bacterium known, Mycoplasma genitalium, is a circular DNA molecule with 580,070 bp. Calculate the molecular weight (assume the molecular weight of a nucleotide pair is 650) and contour length (when relaxed) of this molecule. What is Lk0 for this Mycoplasma chromosome? If σ = −0.06, what is Lk?
Question 9.6
A closed-
A protein complex binds, wrapping the DNA around it to form a solenoidal supercoil.
One DNA strand is broken.
DNA gyrase and ATP are added to the DNA solution.
The double helix is denatured by heat.
Question 9.7
In the presence of a eukaryotic condensin and a type II topoisomerase, the Lk of a relaxed closed-
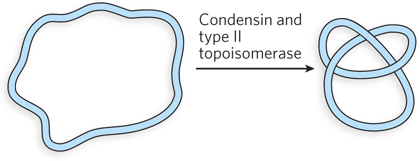
Question 9.8
Bacteriophage λ infects E. coli by integrating its DNA into the bacterial chromosome. The success of this recombination depends on the topology of the E. coli DNA. When the superhelical density (σ) of the E. coli DNA is greater than −0.045, the probability of integration is <20%; when σ is less than −0.06, the probability is >70%. Plasmid DNA isolated from an E. coli culture is found to have a length of 13,800 bp and an Lk of 1,222. Calculate σ for the plasmid DNA (which reflects the superhelical density of all DNA in the cell, plasmid and chromosome), and predict the likelihood that bacteriophage λ will be able to infect this culture.
Question 9.9
What is the Lk of a 5,250 bp circular duplex DNA molecule with a nick in one strand?
What is the Lk of the molecule in (a) when the nick is sealed (forming a relaxed molecule)?
How would the Lk of the molecule in (b) be affected by the action of a single molecule of E. coli topoisomerase I?
What is the Lk of the molecule in (b) after eight enzymatic turnovers by a single molecule of DNA gyrase in the presence of ATP?
What is the Lk of the molecule in (d) after four enzymatic turnovers by a single molecule of bacterial type I topoisomerase?
What is the Lk of the molecule in (d) after binding of one protein that wraps DNA around it to form a solenoidal supercoil, with no other changes in the DNA?
327
Question 9.10
Explain how the underwinding of a B-
Question 9.11
Describe two structural features required for a circular DNA molecule to maintain a negatively supercoiled state.
List three structural conformations that become more favorable when a DNA molecule is negatively supercoiled.
What enzyme, with the aid of ATP, can generate negative supercoiling in DNA?
Describe the physical mechanism by which this enzyme acts.
Question 9.12
YACs are used to clone large pieces of DNA in yeast cells. What three types of DNA sequence are required to ensure proper replication and propagation of a YAC in a yeast cell, and what is the function of each?
Question 9.13
When DNA is subjected to electrophoresis in an agarose gel, shorter molecules migrate faster than longer ones. Closed-
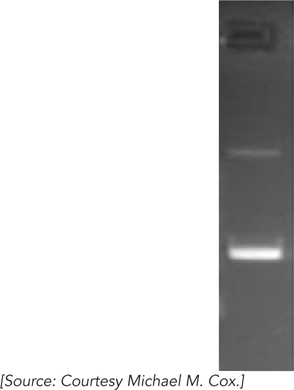
What are the DNA species in the two bands?
If topoisomerase I is added to a solution of this DNA, what will happen to the upper and lower bands after electrophoresis?
If DNA ligase is added to the DNA, will the appearance of the bands change?
If DNA gyrase and ATP are added to the DNA after adding DNA ligase, how will the band pattern change?
Question 9.14
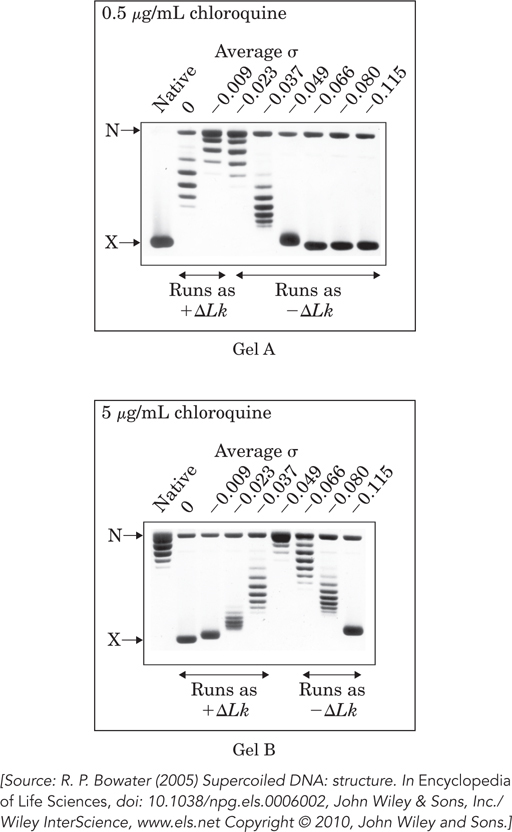
As in the experiment described in Problem 13, gels are run to separate closed-
Chloroquine intercalates between base pairs and stabilizes a more underwound DNA structure. When the dye binds to a relaxed, closed-
328
In gel A, why does the σ = 0 lane (i.e., DNA prepared so that σ = 0, on average) have multiple bands?
In gel B, is the DNA from the σ = 0 preparation positively or negatively supercoiled in the presence of the intercalating dye?
In both gels, the σ = −0.115 lane has two bands, one a highly supercoiled DNA and one relaxed. Propose a reason for the presence of relaxed DNA in these lanes (and others).
The native DNA (leftmost lane in each gel) is the same closed-
circular DNA isolated from bacterial cells and untreated. What is the approximate superhelical density of this native DNA?
Question 9.15
In the early electron microscopy experiments of Vinograd and colleagues (see this chapter’s How We Know section), the polyoma virus DNA was clearly circular in both form I and form II. However, the DNA in form II tended to be spread out on the electron microscope grid, whereas the DNA in form I tended to twist on itself, often repeatedly. Explain these observations.
Question 9.16
A small plasmid DNA is isolated, and one negatively supercoiled topoisomer is purified (see this chapter’s How We Know section). A small amount of bacterial topoisomerase III or topoisomerase II (DNA gyrase) plus ATP is added to the plasmid DNA in two separate experiments, allowing only limited reaction of the topoisomers. The experiments yield the DNA banding patterns shown below. Which pattern is produced by DNA topoisomerase III, and which by DNA gyrase?

Question 9.17
If bacterial topoisomerase I is inactivated by mutating the gene (topA) encoding it, will the superhelical density of the cell’s chromosomal DNA increase or decrease? When such mutations are introduced into the topA gene, compensating mutations appear rapidly in another gene, which serve to partly ameliorate the deleterious cellular effects of the topA mutations. Which gene is likely to be the site of the new mutations, and why?