Budding Yeast, Saccharomyces cerevisiae
Saccharomyces cerevisiae is a microbial ascomycete fungus, used by humans for centuries to make bread, beer, and wine. It is generally referred to by scientists as budding yeast, but is also called yeast, baker’s yeast, and brewer’s yeast. Yeast has several features that make it an attractive model organism. It is a single-
The S. cerevisiae genome is only ∼12 Mbp long, with ∼6,000 genes. Yet it contains homologs to several human disease genes, making it a model for understanding the basic function of gene products involved in some forms of disease, as well as indicating that some human diseases result from the disruption of relatively simple and fundamental life processes. However, the single-
The haploid nature of yeast allows convenient mutational studies. Yeast is also easily transformed with exogenous DNA. This DNA frequently integrates into the chromosome by homologous recombination, making possible the construction of gene knockout strains. The ease of genetics, combined with the ease of growth, makes S. cerevisiae, like E. coli, a model organism that beautifully combines the power of genetics with that of biochemical studies.
Early Studies of Yeast as a Model Organism
Yeast has been a model organism for more than 100 years, and it essentially spawned the field of modern biochemistry. Louis Pasteur proved that yeast ferments sugar to alcohol and CO2; he declared fermentation a vital life force that could not be separated from the living organism. In 1897, Eduard Buchner accidentally observed fermentation in a yeast cell extract, and called the fermenting substance “zymase.” Later studies showed that zymase was actually a mixture of many different enzymes. The suffix ase is used for many enzyme names to this day.
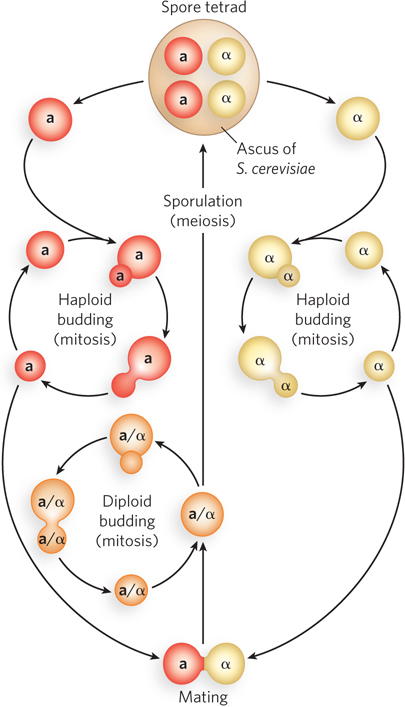
Life Cycle
Budding yeast can exist in either the haploid or the diploid state (Figure A-
Genetic Techniques
Mutagenesis Mutational studies in S. cerevisiae are simplified by its ability to grow as a haploid. This allows the study of mutant phenotypes detectable as failure to grow under diverse conditions (e.g., mutants requiring specific nutritional conditions, or high or low temperature). In the case of gene knockouts, it is very useful that particular nutritional requirements can be made to report the presence or absence of a given knockout.
Complementation Haploid yeast can be mated to produce diploid cells for complementation analysis of mutant haploids. Mutations in essential genes can be maintained by harboring them in the diploid state. On sporulation of a diploid with a mutation in an essential gene, two of the four spores will not be viable. Isolation of all four products of meiosis in an ascus is useful for the analysis of recombination during meiosis.
Introduction of DNA DNA can be introduced into S. cerevisiae by chemical or physical abrasion of the thick cell wall. Linear DNA, with a selectable marker and ends homologous to the yeast chromosome, readily integrates into the genome by homologous recombination. This configuration permits the ends to recombine with their homologous sequence in the yeast chromosome, replacing the gene of interest with the selectable marker and effectively knocking out the gene.
Selectable Markers Selection in yeast is mostly performed with auxotrophs, strains that lack a functional gene for an enzyme in an amino acid biosynthetic pathway and therefore require that amino acid to grow. Cells are grown on defined media lacking the now essential amino acid. Only cells that acquire the marker and thus are capable of synthesizing the amino acid from raw materials will survive. Drug-
Plasmids DNA can also be maintained in yeast as a circular plasmid, using one of the many chromosomal origins known as an ARS (autonomously replicating sequence). YACs (yeast artificial chromosomes) can maintain very large DNA inserts (300 kbp to 1 Mbp), which are useful in genome sequencing projects (see Figure 7-7). S. cerevisae also contains a natural 2 micron (2μ) plasmid, which can be used to maintain exogenously derived DNA inside the cell.
Yeast as a Model Organism Today
Fundamental Mechanisms of Information FlowThe fact that yeast are microbes, the ability to grow them in large quantities for biochemical studies, and the ease of genetic manipulation, all make yeast ideal for studying detailed mechanisms of fundamental processes, including replication, gene regulation, DNA repair, transcription, translation, and recombination.
Cell Division Cycle When yeast divides, the bud is visible in the microscope, as are the size changes at different phases of the cell cycle. Accumulation of mutant cells stuck in a budded or unbudded state has been used as a phenotype by which to identify numerous cell division cycle (cdc) mutants, useful for genetic and biochemical dissection of the cell cycle. Humans have homologs to many of the cell cycle genes discovered in yeast.
Signaling Pathways Yeast is a model for signal transduction pathways. For example, yeast pheromone signaling is a good model for 7-
Meiotic Recombination Because S. cerevisiae produces an ascus with four spores derived from a single cell, recovering all four products permits detection of more complex genetic events than simple crossovers. For example, study of gene conversion in yeast led to an understanding of the role of double-
Systems Biology It is now within the scope of systems biology to understand most of the genetic, physical, and molecular interactions of the 6,000 yeast genes. Pairwise examinations of genetic interactions between these gene knockouts are leading to the identification of new pathways. Proteomic approaches have been pioneered in yeast. Intracellular copy numbers of almost all yeast proteins, proteome-
A-