DNA Replication Is Semiconservative
Watson and Crick hypothesized that during DNA replication, the double helix is unwound and each parental DNA strand is used as a template to generate a new daughter strand, and thus two daughter duplexes, following the A=T and G≡C base-pairing rules. This mode of replication is called semiconservative, because each daughter duplex conserves one strand of parental DNA; the other strand is completely new. However, two other hypotheses were also proposed to explain how DNA could be replicated (Figure 11-1a). In one alternative hypothesis, conservative replication, the two parental strands would remain together while acting as template for production of a new DNA duplex. Another possible mechanism was dispersive replication, in which the duplex would be replicated in a random patchwork.
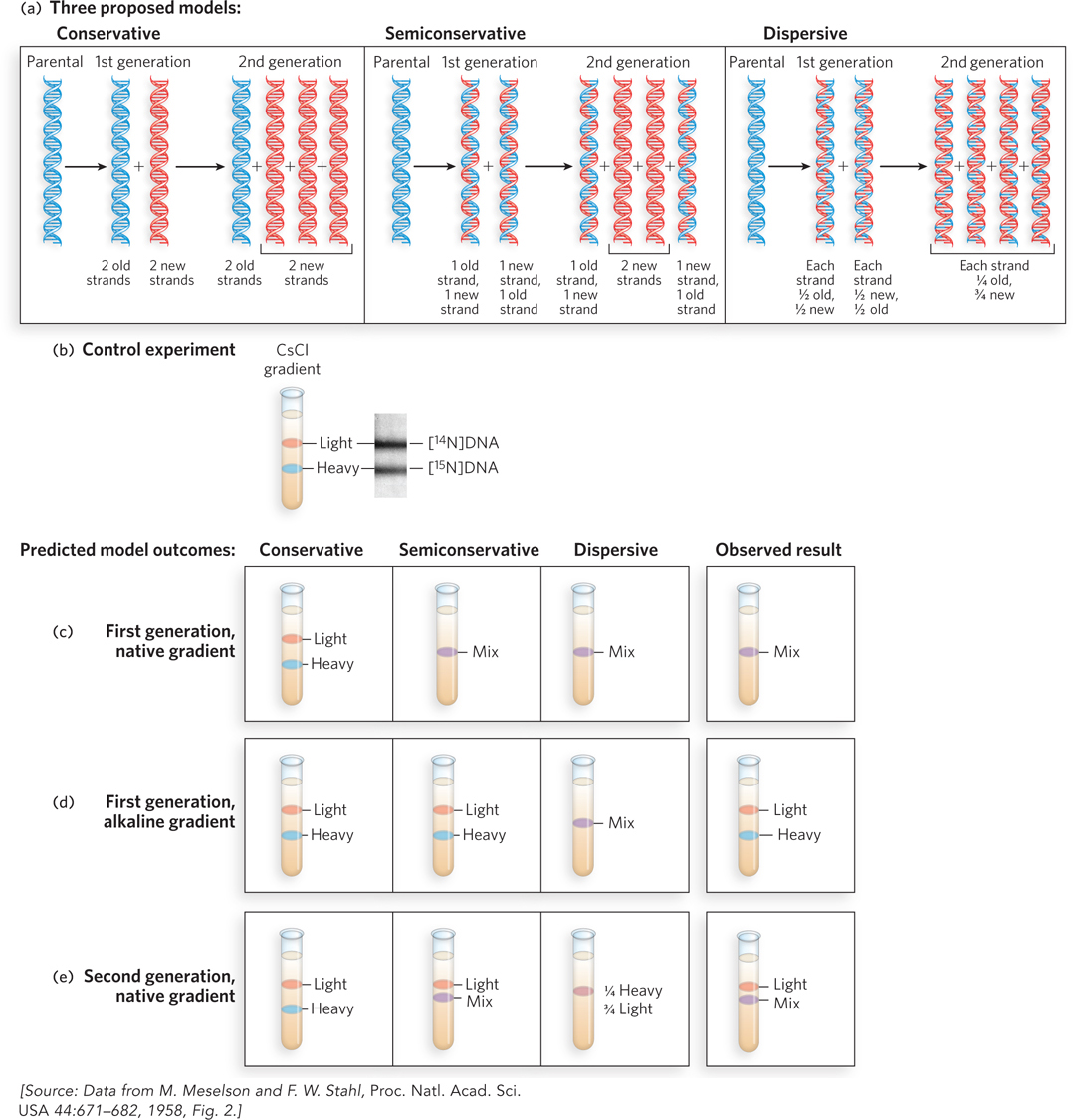
Figure 11-1: Semiconservative DNA replication. (a) Three possible mechanisms of replication, and the distribution of parental DNA (blue) and daughter DNA (red) expected for each, after one and two generations of growth. (b) In one of Meselson and Stahl’s control experiments, equal amounts of double-stranded [14N]DNA (both strands light) and [15N]DNA (both strands heavy) were mixed and subjected to ultracentrifugation in a native CsCl density gradient. At right is a picture of the centrifuge cell, taken while the centrifuge is running, that visualizes DNA bands using UV absorption (DNA absorbs UV light). (c) Expected and observed results in a native CsCl density gradient (DNA is double-stranded) for conservative, semiconservative, and dispersive replication following one round of replication (first generation). (d) Expected and observed results in an alkaline CsCl density gradient (DNA strands separate) after one round of replication. (e) Expected and observed results in a native CsCl density gradient following two rounds of replication (second generation).
An elegant experiment to distinguish among these three replication mechanisms was devised by Matthew Meselson and Franklin Stahl. First, they grew Escherichia coli cells for multiple generations in a medium containing 15NH4 as the sole nitrogen source to uniformly label the cellular DNA with the heavy nitrogen isotope, 15N. Although DNA contains only about 17% nitrogen by mass, and the mass of 15N is only 7% greater than that of 14N (the nitrogen isotope normally found in DNA), the resulting 1.2% increase in the mass of DNA labeled with 15N is enough to separate [15N]DNA from [14N]DNA in a density gradient. Figure 11-1b shows one of their control experiments in which an equal mixture of [14N]DNA and [15N]DNA was added to a solution of CsCl (cesium chloride) in a tube and subjected to ultracentrifugation, forming a density gradient of CsCl from the top to the bottom of the tube. As the density gradient formed, DNA molecules coalesced into sharp bands at positions corresponding to their density (and thus their mass). The “heavy” [15N]DNA formed a band below the band of “light” [14N]DNA.
To analyze DNA replication in cells, 15N-labeled E. coli cells were transferred to unlabeled (i.e., 14N) medium and allowed just one round of replication, then DNA was extracted and analyzed in a CsCl density gradient under either of two conditions: native, in which the two DNA strands of a duplex remain together, or alkaline, in which the duplex separates into two single strands of DNA. The expected results for each of the three hypotheses are shown in Figure 11-1c–e, for native and alkaline CsCl gradients.
We focus first on the expected results for the native CsCl gradient, where DNA remains double-stranded, illustrated in Figure 11-1c. If replication were conservative, two bands of duplex DNA would be observed: one of lower density, containing two new strands of [14N]DNA (light/light), and one of higher density, consisting of the original parental [15N]DNA duplex (heavy/heavy). If replication were semiconservative, only one band of duplex DNA would be observed, each duplex consisting of one parental [15N]DNA strand and one daughter [14N]DNA strand. This heavy/light band of DNA would migrate at a position in the native CsCl gradient that is intermediate between the heavy/heavy and light/light DNA bands. If replication were dispersive, the result observed in a native CsCl gradient would be the same as for the semiconservative model: one band of heavy/light DNA. The actual result of the native CsCl gradient showed only a single band of heavy/light DNA, located between the positions of light/light and heavy/heavy DNA. This result ruled out the conservative model of replication, but did not distinguish between the semiconservative and dispersive models.
The experiment using the alkaline CsCl gradient, in which the two strands of duplex DNA separate, distinguishes between the semiconservative and dispersive models of replication. Following semiconservative replication, the two strands of DNA in a duplex will be very different—one completely heavy [15N]DNA, the other completely light [14N]DNA—and therefore two bands should be observed. In contrast, the two strands of a DNA duplex produced by dispersive replication would both be composed of 50% heavy and 50% light DNA, and thus only one band would be observed and would migrate at the heavy/light position in the alkaline CsCl gradient. The observed results supported the semiconservative mechanism and ruled out the dispersive mechanism.
To be sure of their conclusion, Meselson and Stahl performed one more experiment to distinguish between semiconservative and dispersive replication. Cells were allowed to proceed through a second round of replication in unlabeled medium, and the DNA was again analyzed in a native CsCl gradient. The expected results for each model were as follows. Dispersive replication would produce just one band with a density corresponding to 25% [15N]DNA and 75% [14N]DNA. A very different result was expected for semiconservative replication: the first-generation single band of heavy/light DNA should split into two bands, one of light/light DNA and the other of heavy/light DNA. The observed result showed these two bands, thus confirming the semiconservative mechanism.
Replication Is Initiated at Origins and Proceeds Bidirectionally
Experiments using density gradients could not distinguish whether synthesis initiated at one site or at multiple sites on the chromosome, or whether the sites were defined or random. These questions were answered by observing replication of a circular bacterial plasmid DNA in an electron microscope, using autoradiography. In this procedure, radioactively labeled DNA was extracted and spread on an electron microscope grid, then overlaid with a silver emulsion that formed an image on exposure to radioactivity. The light emitted from the radioactivity in the DNA exposes the silver grains and forms an image called an autoradiograph. In the plasmid experiments, the resulting images of DNA molecules undergoing replication contained a loop or bubble (Figure 11-2). This replication intermediate resembles the Greek letter theta (θ), and this mode of DNA synthesis is often called u-form replication. The images captured in these experiments also revealed that DNA was not completely unwound before it was replicated. Instead, as the strands separated, they were simultaneously replicated to form two sister duplexes. This was evident from the observation that all the DNA strands appear to be the thickness of duplex DNA. Additional experiments showed that the point where DNA synthesis starts was a specific DNA sequence called the origin of replication. The point where the parental duplex separates and the daughter duplexes form was the site of new DNA synthesis, referred to as a replication fork. In bidirectional replication, two replication forks are formed at the origin and propagate away from it in opposite directions. Further studies showed that the circular chromosomes of bacteria are replicated in the same way as plasmids, and bidirectional replication occurs from the origin in the bacterial chromosome as well as in most plasmid DNAs.
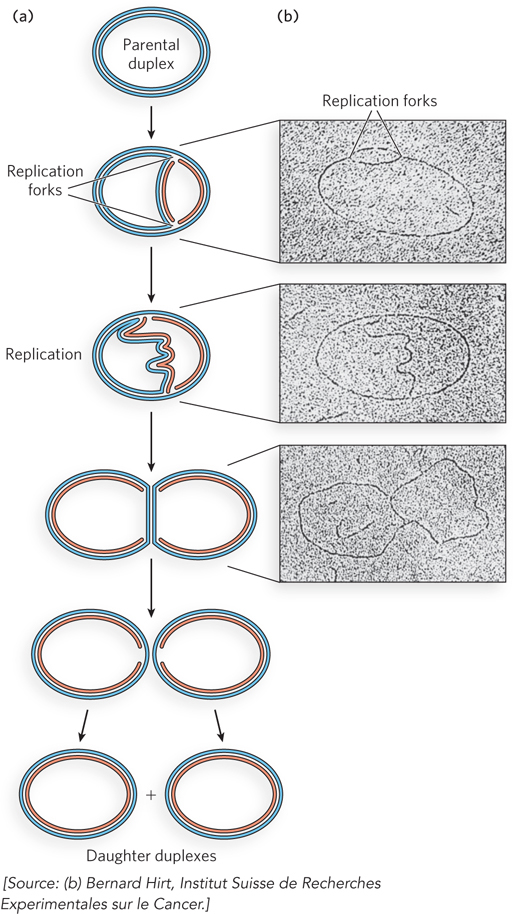
Figure 11-2: Simultaneous replication of both DNA strands. (a) Replication stages of a circular, double-stranded DNA plasmid, based on autoradiography and electron microscopic observations. Blue strands are parental DNA; red strands are new DNA. The two replication forks proceed in opposite directions (bidirectionally) around the circular plasmid until they meet at the opposite side, forming two duplex circles. (b) Autoradiographs viewed in the electron microscope, showing 3H-dTTP-labeled plasmid DNA undergoing replication from a single origin. The origin is located in the center of the small DNA bubble in the first micrograph.
A single origin of replication is sufficient for the comparatively small bacterial chromosome and plasmids, but eukaryotic cells contain much more DNA. Also, eukaryotic replication forks travel at about one-tenth the speed of bacterial replication forks, possibly due to the complex compaction of eukaryotic chromosomes (see Chapters 9 and 10). It would take more than 100 hours to duplicate the DNA in a human cell if each chromosome contained only one replication fork that started from a single origin. To duplicate the larger eukaryotic genomes within a biologically relevant timeframe, numerous replication forks from numerous origins of replication are generated on each chromosome, as shown in experiments using autoradiography (Figure 11-3).
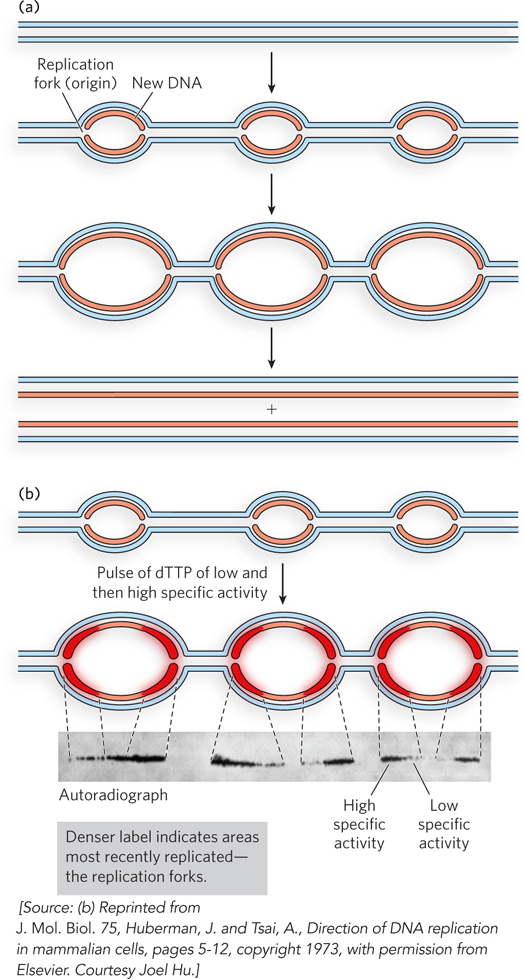
Figure 11-3: Multiple eukaryotic replication forks. (a) In eukaryotes, bidirectional replication forks from multiple origins form replication bubbles that expand and meet head-on to produce two new linear duplex chromosomes. Parental DNA is blue; newly replicated DNA is red. (b) Chinese hamster ovary cells were labeled with two pulses of [3H]dTTP, first of low radioactivity, then high radioactivity. DNA was then extracted and imaged by autoradiography. The image shows the newly replicated, 3H-labeled DNA initiated at multiple origins. Increased density of radioactivity at both ends of a bubble, at the replication forks, reveals that each new DNA strand is growing away from the midpoint and thus that replication is bidirectional. Replication forks had formed prior to addition of [3H]dTTP, so the new DNA is not completely radioactive along its entire length (the DNA closest to the origin is not labeled). On addition of [3H]dTTP, the new DNA becomes labeled. The label intensity increases at each forked junction because of the increase in specific activity of the label during the experiment. Therefore, the taper of the signal in the autoradiograph reveals that the direction of fork movement is bidirectional, away from the midpoint (origin) between two sets of replication forks.
As in bacteria, replication bubbles grow bidirectionally in eukaryotes, with replication forks moving in opposite directions on either side of an origin, as illustrated in Figure 11-3a. An example of how bidirectional replication was tested in mammalian cells is shown in Figure 11-3b. Cells were supplied with 3H-labeled thymidine ([3H]dTTP) in two separate pulses—first with [3H]dTTP having a low level of radioactivity (low specific activity), followed by a short pulse of [3H]dTTP with high radioactivity (high specific activity). The resulting autoradiograph shows a gradient of radioactive density that is heaviest at forked junctions moving in opposite directions, indicating that replication forks travel bidirectionally from each origin. Only the replication bubbles, not the entire DNA, are visible in the autoradiograph, because the duration of the experiment was short—far less than needed for a full round of replication. Experiments of this type have also been performed in bacteria, archaea, and other eukaryotic cells, all with similar results.
We know that specific origin sequences are present in bacteria, archaea, and the simple eukaryote Saccharomyces cerevisiae (baker’s yeast), but the nature of the origins of replication in complex eukaryotes remains uncertain. More details on replication origins, including how they initiate replication, are given in Section 11.4.
Bidirectional replication is the most common form of replication, but not completely universal. The E. coli Col E1 plasmid is an example of a circular plasmid with a defined origin that replicates in a single direction. The genomes of some organisms, such as bacteriophage Φ29 and adenovirus, are single, linear, double-stranded DNAs with no internal origin. These genomes are replicated starting from their ends. Another form of replication, used by certain phages with a circular genome, involves a single replication fork that proceeds multiple times around the circular DNA to generate numerous copies of the genome.
Replication Is Semidiscontinuous
Now that we have seen that replication occurs bidirectionally with one replication fork at each end of the bubble, we take a closer look at how two daughter strands are produced at a single replication fork. The Y-shaped replication fork consists of a parental duplex DNA stem and two prongs, the new daughter duplexes. The parental DNA strands are antiparallel, so the links between nucleotides in the two daughter strands also run in opposite directions (Figure 11-4). However, all known DNA polymerases, the enzymes that synthesize DNA, extend DNA in only one direction: 5′→3′. Hence, both daughter strands cannot be replicated in the same direction in which the replication fork moves. Furthermore, as we discuss later in the chapter, DNA polymerase cannot initiate DNA chains; these must be initiated by short sections of RNA or DNA, referred to as primers. In the cell, the primers at a replication fork are RNA, synthesized by an enzyme called primase.
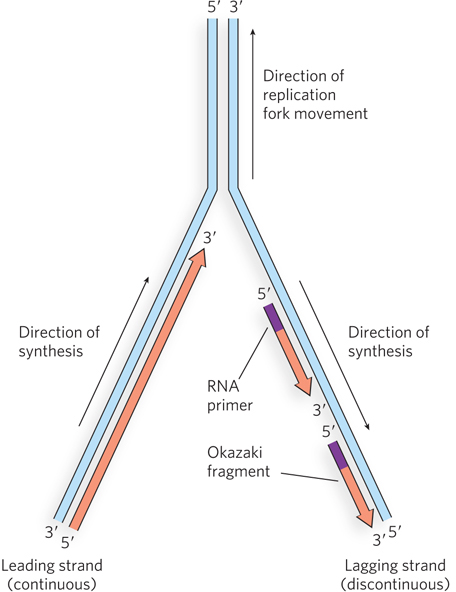
Figure 11-4: Structure of the replication fork. DNA polymerases can extend DNA only in the 5′→3′ direction, but the two parental strands are antiparallel. Therefore, one daughter strand (the leading strand) is synthesized continuously, in the direction of fork movement, while the strand synthesized in the opposite direction (the lagging strand) must be replicated discontinuously as a series of Okazaki fragments.
KEY CONVENTION
The direction of synthesis by DNA polymerases refers to the direction in which each new nucleotide is chemically linked to the growing daughter strand. All DNA polymerases function in the 5′→3′ direction, linking the α-5′-phosphate of a new dNTP to the 3′ position of the nucleotide residue at the end (i.e., the 3′ end) of the chain.
Reiji Okazaki and his coworkers investigated the proposal that one of the daughter strands is continuously extended in the same direction in which the replication fork moves, while the other strand is synthesized in the opposite direction. The strand that is made in the direction opposite to fork movement is still synthesized in the 5′→3′ direction, and thus the strand must be reinitiated at intervals and synthesized as a series of fragments (see Figure 11-4). This mode of replication is described as semidiscontinuous, because only one daughter strand is synthesized continuously; the other is made as a series of discontinuous fragments. The continuously synthesized daughter strand is called the leading strand, and the discontinuous daughter strand is the lagging strand. The “lagging” designation is based on the fact that some unreplicated single-stranded DNA is generated on the lagging strand by the moving fork, so the conversion to duplex DNA on this strand lags behind that of the leading strand.
The model of semidiscontinuous replication predicts that during chromosome replication, short DNA fragments are produced on one of the strands. These fragments are expected to exist only transiently before being connected together. To look for these lagging-strand fragments, Okazaki used E. coli cells infected with T4 phage. Because T4 makes many copies of itself simultaneously, detection of lagging-strand fragments is made easier by their abundance. T4-infected E. coli cells were labeled with radioactive nucleotide precursors for brief time intervals, then the DNA was analyzed in alkaline CsCl gradients to separate the new, radioactive DNA strands from the unlabeled parental strands. Small fragments in the 1,000 to 2,000 nucleotide (1 to 2 kb) range were observed, as predicted, and have come to be known as Okazaki fragments. Lagging-strand Okazaki fragments are 1 to 2 kb long in bacteria, but are shorter—only 100 to 200 nucleotides—in eukaryotes. Each Okazaki fragment is primed at the 5′ end by a short RNA of 10 to 13 nucleotides. As we discuss in detail later in the chapter, the RNA primer is removed by nuclease action, and the Okazaki fragments are joined by ligase soon after the replication fork has passed.
SECTION 11.1 SUMMARY
DNA replication is semiconservative; each daughter chromosome contains one parental strand and one newly synthesized strand.
Replication is initiated at specific sites on the DNA, the origins of replication. Circular bacterial chromosomes usually have only one origin, whereas long, linear eukaryotic chromosomes have numerous origins.
Replication usually proceeds bidirectionally from the origin, at growing points known as replication forks.
At a replication fork, the parental DNA strands are separated and used as templates to simultaneously form two new daughter duplexes.
The two strands of DNA in a duplex are antiparallel, yet DNA polymerases extend DNA only in the 5′→3′ direction. Therefore, only one strand, the leading strand, is extended “continuously” in the direction of replication fork movement; the other strand, the lagging strand, is synthesized “discontinuously” in the opposite direction as a series of Okazaki fragments, which subsequently are joined by ligase. This is the process of semidiscontinuous replication.



