16.4 PROCESSING OF NON-PROTEIN-CODING RNAs
We have seen how the cell produces mature mRNA transcripts through a series of processing mechanisms. Each aspect of mRNA processing, transport, and decay is carefully regulated to control how, when, and where the mRNA is made or translated. RNA processing is not unique to mRNAs. All functional RNAs in cells are produced as precursor transcripts that must be cleaved to form the mature, functional RNA. This processing could be required because RNA polymerases do not always have specific termination sites, so transcripts from the same gene can have heterogeneous ends. Processing also provides convenient opportunities for the cell to regulate RNA levels. We discuss here the processing of three different kinds of functional RNAs central to gene expression: transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), and miRNAs.
Maturation of tRNAs Involves Site-Specific Cleavage and Chemical Modification
Transfer RNAs, the molecules that carry amino acids to the ribosome during protein synthesis (see Chapter 18), are coordinately expressed in response to metabolic needs. In some cases, multiple tRNAs are synthesized as a single primary transcript and are separated by enzymatic cleavage. Even tRNAs synthesized alone are derived from longer RNA precursors by the enzymatic removal of nucleotides from the 5′ and 3′ ends (Figure 16-27). The endonuclease ribonuclease P (RNase P), a ribonucleoprotein found in all organisms, removes nucleotides from the 5′ end of tRNAs. The RNA component of the enzyme is essential for activity. Indeed, bacterial RNase P can carry out its processing function with precision even in the absence of the protein component. The 3′ end of tRNAs is processed by one or more nucleases, including the exonuclease RNase D.
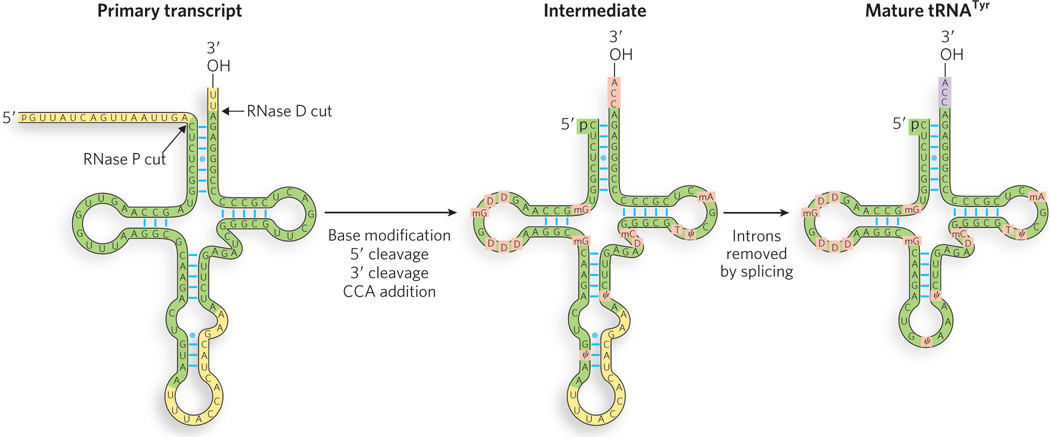
Figure 16-27: The processing of tRNA. Transfer RNAs are trimmed at the 5′ and 3′ ends by RNase P and RNase D, respectively. Shown here is the processing of tRNATyr. The CCA trinucleotide is added to the 3′ end, and some bases are modified to provide stability and enhance the functioning of the mature tRNA. In some eukaryotic tRNAs, introns are excised during processing.
In eukaryotes, a few tRNA transcripts have introns that must be excised. These introns are spliced by an ATP-dependent mechanism distinct from that of the spliceosome: a splicing endonuclease that recognizes and cleaves the phosphodiester bonds at the intron splice sites. RNA ligase joins the two exons to complete the reaction.
Transfer RNA precursors often undergo further posttranscriptional processing. The terminal CCA-3′ to which an amino acid is attached is absent from some bacterial and all eukaryotic tRNA precursors, and is added after the initial transcript is made (see Figure 16-27). This addition is carried out by tRNA nucleotidyltransferase, an unusual enzyme that binds the three ribonucleoside triphosphate precursors in separate active sites and catalyzes the formation of the phosphodiester bonds to produce the CCA-3′ sequence. The creation of this defined sequence of nucleotides is therefore not dependent on a DNA or RNA template—the template consists of the three binding sites of the nucleotidyltransferase.
The final type of tRNA processing is the modification of some bases by methylation, deamination, or reduction. In some cases, a base is removed from a specific site in the tRNA sequence and replaced by a different, noncanonical base (Figure 16-28). For example, in some tRNAs, uracil is removed at a particular U residue and reattached to the ribose through C-5 to create pseudouridine (ψ). Modified bases often occur at characteristic positions in all tRNAs, suggesting their importance for structural stability or recognition of the tRNA by other enzymes (see the How We Know section at the end of this chapter).
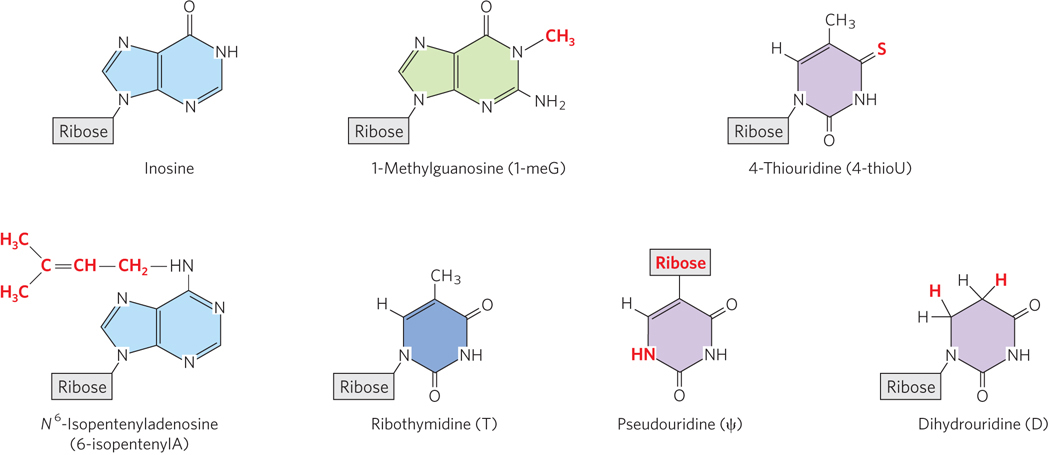
Figure 16-28: Modified tRNA bases produced in posttranscriptional reactions. Specific nucleotide residues in tRNAs, as well as in other RNAs including rRNAs, are chemically modified by enzymes that recognize particular structures and/or sequences.
Maturation of rRNA Involves Site-Specific Cleavage and Chemical Modification
Ribosomal RNAs of bacterial and eukaryotic cells are produced from longer precursors called preribosomal RNAs (pre-rRNAs). The pre-rRNA transcripts, produced by RNA polymerase I (Pol I) in eukaryotes, are coordinately synthesized so that they are present in similar amounts for ribosome assembly. In bacteria, the three rRNAs needed to form a functional ribosome—16S, 23S, and 5S—arise from a single 30S RNA precursor of about 6,500 nucleotides. RNA at both ends of the 30S precursor and segments between the rRNAs are removed during processing (Figure 16-29).
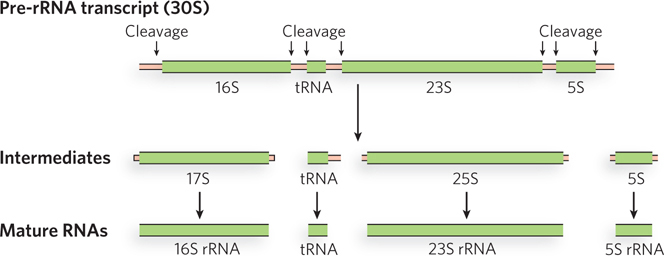
Figure 16-29: Processing of pre-rRNA transcripts in bacteria. The 30S pre-rRNA contains the 16S, 23S, and 5S rRNA sequences, as well as a tRNA sequence. During processing, these segments are separated by nuclease activities.
KEY CONVENTION
For historical reasons stemming from their initial discovery as large, polymeric components of ribosomes, rRNAs are named according to their sedimentation behavior during ultracentrifugation. Thus, 16S refers to 16 svedberg units (S), named for the Swedish physicist Theodor Svedberg (1884–1971) who invented ultracentrifugation. A svedberg describes the sedimentation properties of particles during centrifugation and is defined as exactly 10−13 seconds.
The E. coli genome encodes seven pre-rRNA molecules, each with essentially identical rRNA-coding regions but differing in the segments between these regions. One or two tRNA genes are found between the 16S and 23S rRNA genes, with different tRNAs arising from different pre-rRNA transcripts. Coding sequences for tRNAs are also found on the 3′ side of the 5S rRNA in some precursor transcripts.
In eukaryotes, a 45S pre-rRNA transcript is processed in the nucleolus to form the 18S, 28S, and 5.8S rRNAs characteristic of eukaryotic ribosomes. In an interesting quirk of evolution, the 5S rRNA of most eukaryotes is made as a completely separate transcript by a different polymerase (Pol III, rather than Pol I). The enzymes responsible for rRNA processing are localized in the nucleolus and are thought to begin cleaving the pre-rRNA transcript during transcription. Processing occurs in an ordered set of reactions guided in part by RNAs in small nucleolar ribonucleoproteins (snoRNPs), which base-pair with the pre-rRNA transcript at specific cleavage sites. The binding of snoRNPs also specifies sites where methyl groups are added to 2′-hydroxyl groups in rRNAs. Careful experiments comparing the function of rRNAs with and without particular sites methylated have failed to reveal a dramatic difference in behavior. However, bacteria or yeast cells with all of the naturally occurring sites in rRNA methylated are found to outcompete cells lacking some of these sites.
Small Regulatory RNAs Are Derived from Larger Precursor Transcripts
Most eukaryotic cells use RNAs called microRNAs (miRNAs) (21 to 23 nucleotides) to regulate the expression of many genes. In addition, many eukaryotes also use short interfering RNAs (siRNAs) to prevent protein synthesis from specific mRNAs. These mechanisms of regulation by miRNAs and siRNAs, known as RNA interference, are discussed in detail in Chapter 22.
The miRNAs, like tRNAs and rRNAs, are encoded by genes that are transcribed but not translated into protein. Primary miRNA transcripts (pri-miRNAs) are typically synthesized by Pol II and then capped and polyadenylated. These transcripts have the ability to fold into extended hairpinlike structures with extended single-stranded RNA on the 5′ and 3′ sides of the hairpin. Such structures are recognized by an RNA-binding protein, which in humans is encoded by DGCR8 (the DiGeorge syndrome critical region gene 8). The DGCR8 protein recruits nuclear pri-miRNAs to the enzyme Drosha to form a microprocessor complex (Figure 16-30). Drosha is an endonuclease that cleaves the pri-miRNA hairpin duplex to produce precursor miRNAs (pre-miRNAs), ∼70 nucleotides in length and containing characteristic dinucleotide 3′ overhangs. Pre-miRNAs then assemble with RNA export proteins, including exportin-5, for transport out of the nucleus. Most pre-miRNAs are not perfectly base-paired hairpins, but instead contain one or more unpaired bases, or non-Watson-Crick base pairs, in the stem of the hairpin—perhaps because perfectly double-stranded RNAs would activate the cell’s antiviral machinery through a pathway known as the interferon response.
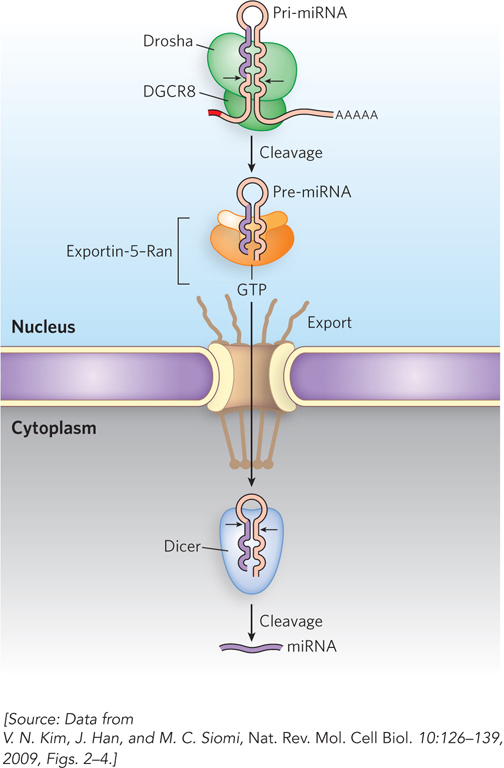
Figure 16-30: Processing of small regulatory RNAs. The small miRNAs and siRNAs are formed by the processing of much larger precursor transcripts by specific endonucleases.
Once in the cytoplasm, pre-miRNAs are further cleaved by the double-stranded RNA endonuclease called Dicer, releasing the mature miRNA. Dicer recognizes the dinucleotide overhangs of pre-miRNAs, which help position RNA substrates such that Dicer’s cleavage products have a characteristic length of 21 to 23 bp. Dicer can also cleave long double-stranded RNAs generated within the cell or introduced by viral infection or transfection, to produce siRNAs. Dicer assists in loading miRNAs and siRNAs into complexes called RNA-induced silencing complexes (RISCs). Mature miRNAs and siRNAs can base-pair with complementary sequences in the 3′UTRs of specific mRNAs to induce degradation and/or translational silencing of those mRNAs.
SECTION 16.4 SUMMARY
Transfer RNAs are produced from precursor transcripts that are enzymatically cleaved at the 5′ and 3′ ends. In some cases, internal sequences are excised and the flanking sequences ligated to produce the mature tRNA.
Transfer RNAs contain unusual bases or nucleotide modifications that are formed posttranscriptionally.
Ribosomal RNAs are produced from much longer precursor transcripts by site-specific cleavage, and particular nucleotides are chemically modified.
Regulatory RNAs, such as miRNAs, are also processed from larger transcripts.



