18.4 ELONGATION AND TERMINATION OF THE POLYPEPTIDE CHAIN
In the second stage of protein synthesis, elongation, the nascent polypeptide is lengthened by the covalent attachment of successive amino acid units; each unit is carried to the ribosome and correctly positioned by its tRNA, which base-pairs to its corresponding codon in the mRNA. The binding of each incoming aminoacyl-tRNA and the movement of the ribosome along the mRNA are facilitated by the hydrolysis of GTP as each residue is added to the growing polypeptide. These steps are generally the same in bacteria and eukaryotes: just the names of some elongation factors differ. Because bacterial elongation is better defined, we describe that process here, focusing mainly on formation of the first peptide bond: conversion of the fMet-tRNA to a dipeptidyl-tRNA. The completion of polypeptide synthesis, termination, is signaled by one of three mRNA codons (UAA, UAG, and UGA) that act as termination codons, or stop codons. As the translation product is released, the ribosomes and associated factors can be recycled for another round of translation. After discussing the events of elongation and termination, we consider the overall energy requirements of protein synthesis and some effects of antibiotics on the protein synthesis machinery.
Peptide Bonds Are Formed in the Translation Elongation Stage
In bacteria, elongation requires the initiation complex described in Section 18.3, aminoacyl-tRNAs, GTP, and several elongation factors: EF-Tu, EF-Ts, and EF-G.
Cells use three steps to add each amino acid residue, and the steps are repeated as many times as there are residues to be added, as summarized in Figure 18-22. In step 1, the appropriate incoming aminoacyl-tRNA binds to the ribosome with the help of EF-Tu, the most abundant protein in an E. coli cell (Figure 18-23). The aminoacyl-tRNA binds first to a complex of GTP-bound EF-Tu, which protects the amino acid–tRNA linkage from hydrolysis, and then to the A site of the 70S initiation complex. Next, the GTP is hydrolyzed and an EF-Tu–GDP complex is released from the 70S ribosome. The EF-Tu–GTP complex is regenerated in a process involving EF-Ts and GTP.
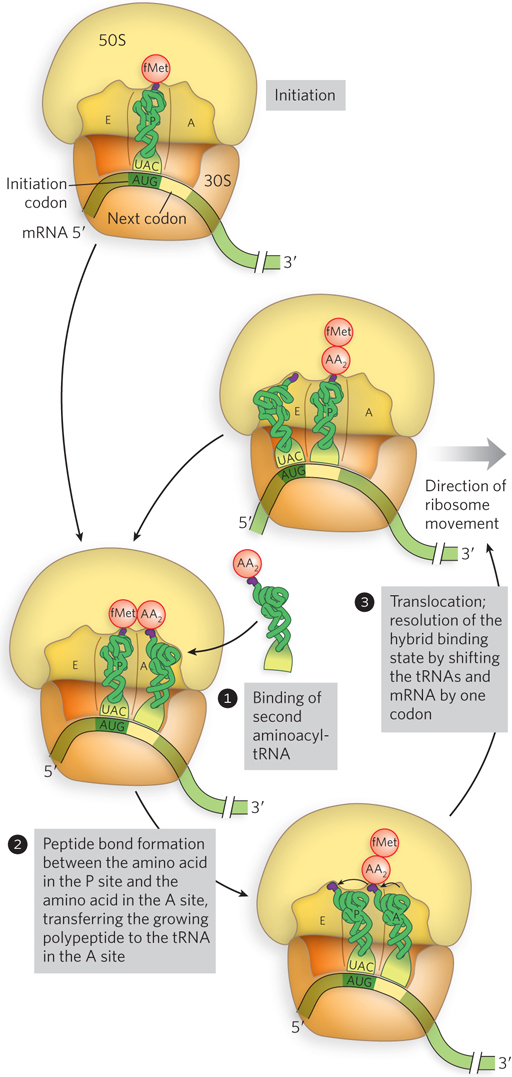
Figure 18-22: An overview of the translation elongation cycle. The details of these three steps are shown in Figures 18–23, 18–24, and 18–26.
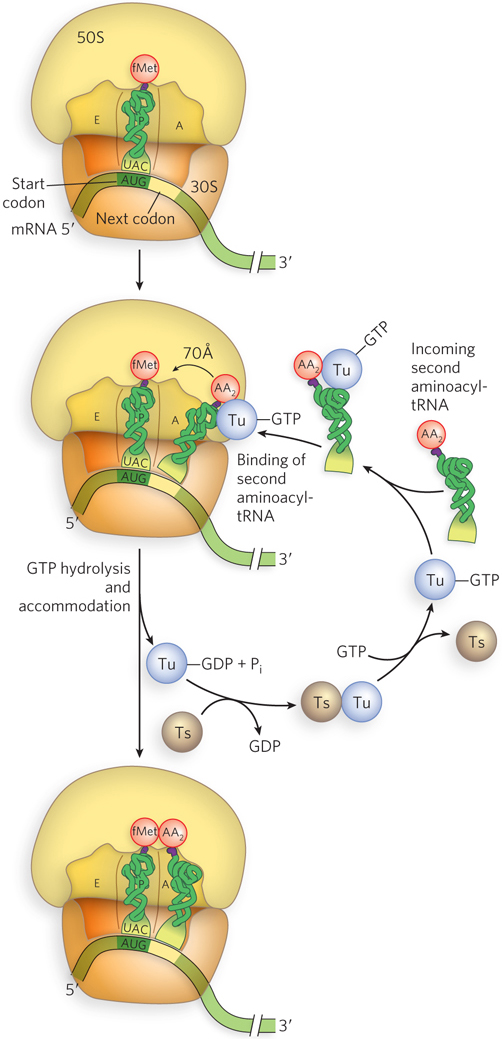
Figure 18-23: EF-Tu–mediated binding of aminoacyl-tRNA to the ribosome. In the first step of elongation, the incoming aminoacyl-tRNA is bound by EF-Tu–GTP and inserted into the A site (EF-Tu is shown simply as Tu). GTP hydrolysis releases EF-Tu–GDP, leaving the tRNA in place. Through accommodation, the tRNA base-pairs with the mRNA codon and shifts by 70 Å into the correct position for the peptidyl transferase reaction. EF-Ts (shown as Ts) recycles EF-Tu by regenerating the EF-Tu–GTP complex.
The GTPase activity of EF-Tu during the first step of elongation in bacterial cells makes an important contribution to the rate and fidelity of protein synthesis. Both the EF-Tu–GTP and the EF-Tu–GDP exist for a few milliseconds before they dissociate. These two intervals provide opportunities for proofreading of the codon-anticodon interactions. Once EF-Tu–GTP is hydrolyzed to EF-Tu–GDP, it loses binding affinity for the aminoacyl-tRNA. When EF-Tu–GDP releases the aminoacyl end of the tRNA within the ribosome, that end is still far from the active site of peptide bond formation. Correct codon-anticodon interactions in the ribosome rotate the tRNA into position for reaction with the growing polypeptide chain (or with the fMet group, for formation of the first peptide bond), in a process called accommodation. Incorrect aminoacyl-tRNAs normally dissociate from the A site at this time. If, in an in vitro experiment, the GTP analog guanosine 5′-O-(3-thiotriphosphate) (GTPγS) is used in place of GTP, hydrolysis is slowed, improving the fidelity (by increasing the proofreading interval) but reducing the rate of protein synthesis.
The process of protein synthesis (including the characteristics of codon-anticodon pairing) has been optimized through evolution to balance the requirements for both speed and fidelity. Improved fidelity might diminish speed, whereas increased speed would probably compromise fidelity. Notice that the proofreading mechanism on the ribosome establishes only that the proper codon-anticodon pairing has taken place. As we saw in Section 18.2, the identity of the amino acid attached to a tRNA is not checked on the ribosome.
Substrate Positioning and the Incoming tRNA Contribute to Peptide Bond Formation
In the second step of elongation (Figure 8-18, step 2), a peptide bond is formed between the two amino acids bound by their tRNAs to the A and P sites on the ribosome (Figure 18-24). Formation of the first peptide bond occurs by the transfer of the initiating N-formylmethionyl group from its tRNA in the P site to the amino group of the second amino acid on its tRNA in the A site. The α-amino group of the amino acid in the A site acts as a nucleophile, displacing the tRNA in the P site to form the peptide bond. This reaction produces a dipeptidyl-tRNA in the A site, leaving an uncharged (deacylated) tRNAfMet bound to the P site. The tRNAs then shift to a hybrid binding state, with elements of each spanning two different sites on the ribosome. To resolve this hybrid binding state, in the third, or translocation, step of elongation (described in detail below), the ribosome moves down the mRNA one codon, so the deacylated tRNAfMet is repositioned to the E site, and the dipeptidyl-tRNA is repositioned to the P site, making the A site available for another aminoacyl-tRNA.
The enzyme that catalyzes peptide bond formation, peptidyl transferase, is intrinsic to the 23S rRNA of the large ribosomal subunit. The peptidyl transferase reaction entails nucleophilic attack of the α-amino group of the A-site aminoacyl-tRNA on the carbonyl carbon of the ester bond linking the fMet (or the growing peptide chain) to the P-site tRNA (see Figure 18-24). The ribosome increases the rate of peptide bond formation 106- to 107-fold above the intrinsic (uncatalyzed) rate of ∼10−4 m−1s−1. Combined evidence from x-ray crystallographic, genetic, and biochemical studies supports the idea that the A- and P-site substrates are precisely aligned in the active site by interactions of both the 3′-terminal CCA sequences of the tRNAs and the nucleophilic α-amino group with nucleotides in the 23S rRNA.
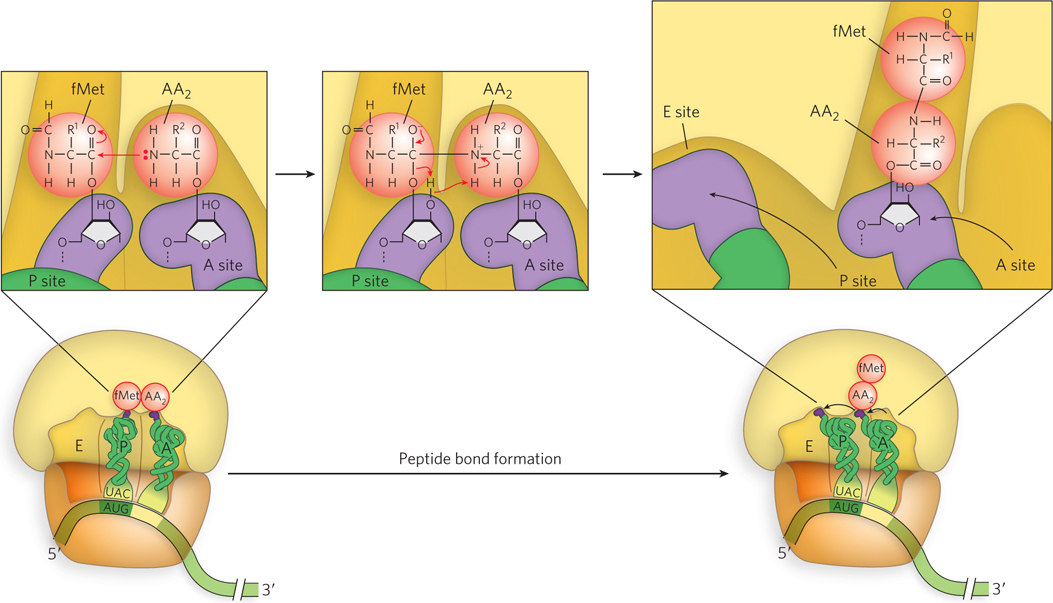
Figure 18-24: The peptidyl transferase reaction. In the second step of elongation, the α-amino group of the A-site aminoacyl-tRNA (shown here as the uncharged –NH2 group) attacks the carbonyl carbon of the P-site fMet-tRNA, shifting the fMet to the A-site tRNA to form a dipeptidyl-tRNA. This reaction is catalyzed by the 23S rRNA.
The proposed catalytic pathway of the peptidyl transferase reaction involves a six-membered transition state in which proton shuttling occurs by way of the 2′-OH of the terminal A residue (A76) of the P-site tRNA. In addition to the close positioning and orientation of the reactive groups relative to each other, the ribosome provides an electrostatic environment that reduces the energetic cost of forming the highly polar transition state by shielding the reaction from bulk water. In this organized environment, the ribosome prevents the extensive solvent rearrangement and consequent decrease in entropy that would occur if the same reaction took place in solution. In this way, the reaction on the ribosome is driven by a favorable entropy change.
EF-G Drives Translocation by Displacing the A-Site tRNA
Immediately following formation of the first peptide bond, the ribosome moves one codon toward the 3′ end of the mRNA, in a process called translocation (see Figure 18-22, step 3). This movement shifts the anticodon of the dipeptidyl-tRNA, which is still attached to the second codon of the mRNA, from the A site to the P site, and shifts the deacylated tRNA from the P site to the E site, from which the tRNA is released into the cytosol. The third codon of the mRNA now lies in the A site and the second codon in the P site.
Movement of the ribosome along the mRNA requires the energy provided by hydrolysis of another molecule of GTP by the GTPase EF-G. EF-G is similar in structure to the EF-Tu–aminoacyl-tRNA complex (Figure 18-25) and can bind to the large subunit side of the A site, displacing the peptidyl-tRNA. On binding of EF-G–GTP to the A site, interactions with a region of the large subunit trigger GTP hydrolysis (Figure 18-26). When GTP is hydrolyzed, the EF-G structure changes so that it can make contact with the small subunit and bring about translocation of the A-site tRNA. It is thought that the change in EF-G structure as GTP is hydrolyzed leads to a change in the three-dimensional conformation of the entire ribosome, resulting in its movement along the mRNA.
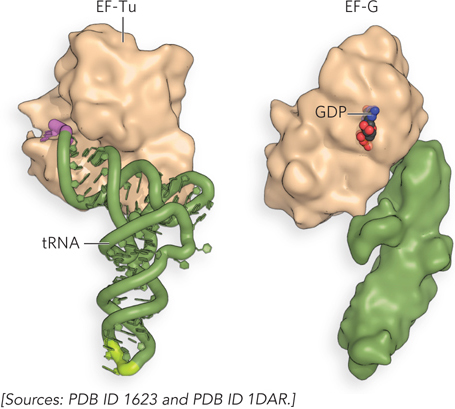
Figure 18-25: EF-Tu–aminoacyl-tRNA and EF-G. The structure of EF-G mimics that of the EF-Tu–aminoacyl-tRNA complex, allowing EF-G to fit in the A site.
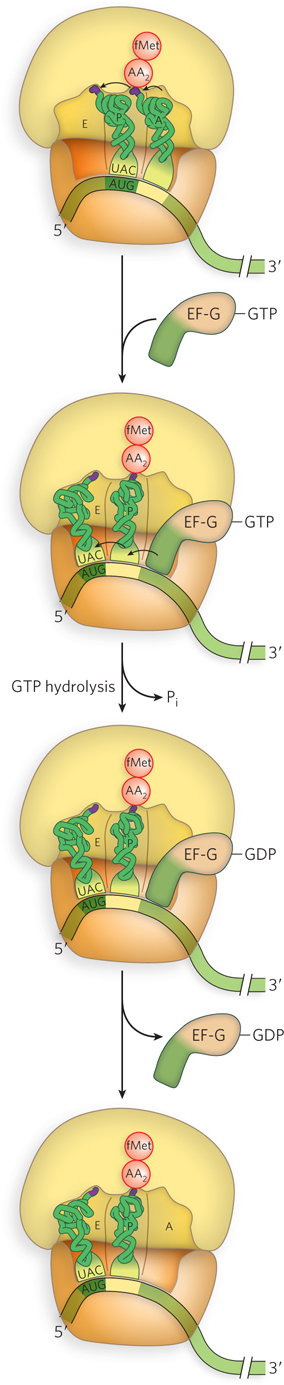
Figure 18-26: Translocation promoted by EF-G binding and GTP hydrolysis. In the third step of elongation, the hybrid binding state formed in the peptidyl transferase reaction (see Figure 18-24) is resolved by the binding of EF-G–GTP at the A site. GTP hydrolysis causes a conformational change in EF-G that displaces the A-site peptidyl-tRNA into the P site, causing the ribosome to move forward by one codon on the mRNA. Once EF-G–GDP is released, the A site is available for another aminoacyl-tRNA.
GTP Binding and Hydrolysis Regulate Successive Elongation Cycles
The ribosome, with its attached dipeptidyl-tRNA and mRNA, is now ready for addition of a third amino acid residue and the next elongation cycle. This process occurs in the same way as addition of the second residue: EF-Tu–GTP delivers a charged tRNA to the A site, the rRNA catalyzes peptide bond formation, then EF-G–GTP displaces the peptidyl-tRNA into the P site. For each amino acid residue correctly added to the growing polypeptide, two GTPs are hydrolyzed to GDP and Pi as the ribosome moves from codon to codon along the mRNA toward the 3′ end.
The polypeptide remains attached to the tRNA of the most recent amino acid to be inserted. This association maintains the functional connection between the information in the mRNA and its polypeptide product. At the same time, the ester linkage between this tRNA and the C-terminus of the growing polypeptide activates the terminal carboxyl group for nucleophilic attack by the incoming amino acid to form a new peptide bond. As the existing ester linkage between the polypeptide and tRNA is broken during peptide bond formation, the linkage between the polypeptide and the information in the mRNA persists, because each newly added amino acid is still attached to its tRNA.
The elongation cycle in eukaryotes is quite similar to that in bacteria. Three eukaryotic elongation factors—eEF1α, eEF1βγ, and eEF2—have functions analogous to those of the bacterial elongation factors EF-Tu, EF-Ts, and EF-G, respectively.
An mRNA Stop Codon Signals Completion of a Polypeptide Chain
Elongation continues until the ribosome adds the last amino acid coded by the mRNA. Termination is signaled by the presence of a stop codon in the mRNA. Mutations in a tRNA anticodon that allow an amino acid to be inserted at a termination codon are generally deleterious to the cell.
In bacteria, once a stop codon occupies the ribosomal A site, three termination factors, or release factors—the proteins RF-1, RF-2, and RF-3—contribute to (1) hydrolysis of the terminal peptidyl-tRNA bond; (2) release of the free polypeptide and the last tRNA, now uncharged, from the P site; and (3) dissociation of the 70S ribosome into its 30S and 50S subunits, ready to start a new cycle (Figure 18-27). RF-1 and RF-2 are related factors and are referred to as class I release factors; RF-3 acts differently and is referred to as a class II release factor. RF-1 and RF-2 recognize termination codons and bind the ribosome in much the same way as tRNAs. RF-1 recognizes stop codons UAG and UAA, and RF-2 recognizes UGA and UAA. Either RF-1 or RF-2 (depending on which codon is present) binds at the stop codon and induces peptidyl transferase to transfer the growing polypeptide to a water molecule rather than to another amino acid.
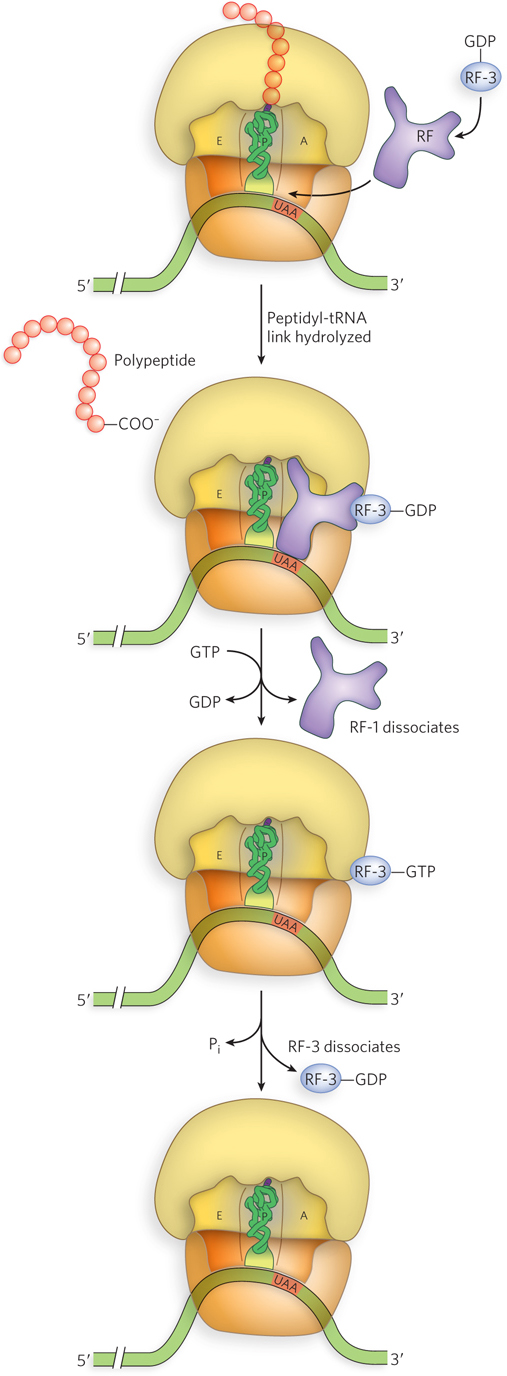
Figure 18-27: Termination of translation in bacteria. When the ribosome comes to a stop codon, RF-1 or RF-2 (designated here by RF) binds to the A site and induces release of the polypeptide chain. RF-3–GDP then binds to the ribosome and exchanges GTP for GDP, displacing the RF-1 or RF-2. The RF-3–GTP is tightly bound to the ribosome, but GTP hydrolysis weakens its affinity and RF-3 is released.
RF-1 and RF-2 have domains that mimic the structure of a tRNA (Figure 18-28). RF-3, a GTPase, catalyzes the dissociation of RF-1 and RF-2 from the ribosome following release of the polypeptide chain. Unlike the other GTP-binding factors that regulate translation, RF-3 binds with higher affinity to GDP than to GTP. For this reason, RF-3–GDP is the predominant form of the factor that binds initially to the ribosome. However, the association between RF-3–GDP and the ribosome is weak unless one of the other release factors, RF-1 or RF-2, is present. On polypeptide release, stimulated by RF-1 or RF-2, an associated ribosomal conformational change triggers exchange of the RF-3–bound GDP for GTP. The RF-3–GTP has a much higher affinity for the ribosome, leading to displacement of RF-1 or RF-2 and creating contact between RF-3 and the factor-binding center of the large ribosomal subunit. As observed for other GTPases that bind the ribosome, this interaction on the large subunit stimulates GTP hydrolysis, allowing RF-3–GDP to fall off.
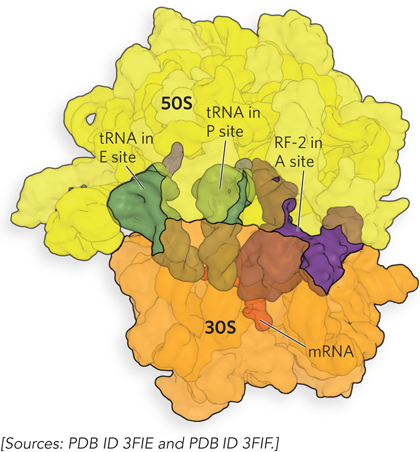
Figure 18-28: The crystal structure of RF-2 bound in the ribosomal A site. RF-1 (not shown) and RF-2 are proteins that mimic tRNAs, binding to the A site and mRNA, displacing the last tRNA from the A site into the P site, and releasing the polypeptide from the ribosome.
In eukaryotes, a single release factor, eRF1, recognizes all three termination codons. A second release factor, eRF3, catalyzes GTP-dependent release of eRF1 from the ribosome.
Ribosome Recycling Factor Prepares Ribosomes for New Rounds of Translation
After release of the polypeptide and the termination factors, the ribosome remains bound to the mRNA and contains deacylated tRNA in the P and E sites. Ribosome recycling removes the mRNA and tRNAs and separates the ribosome into its subunits in preparation for new rounds of translation. In bacteria, ribosome recycling factor (RRF) binds to the empty ribosomal A site and recruits EF-G to stimulate release of the uncharged tRNAs in the P and E sites, in a process mimicking EF-G–stimulated polypeptide elongation (Figure 18-29).
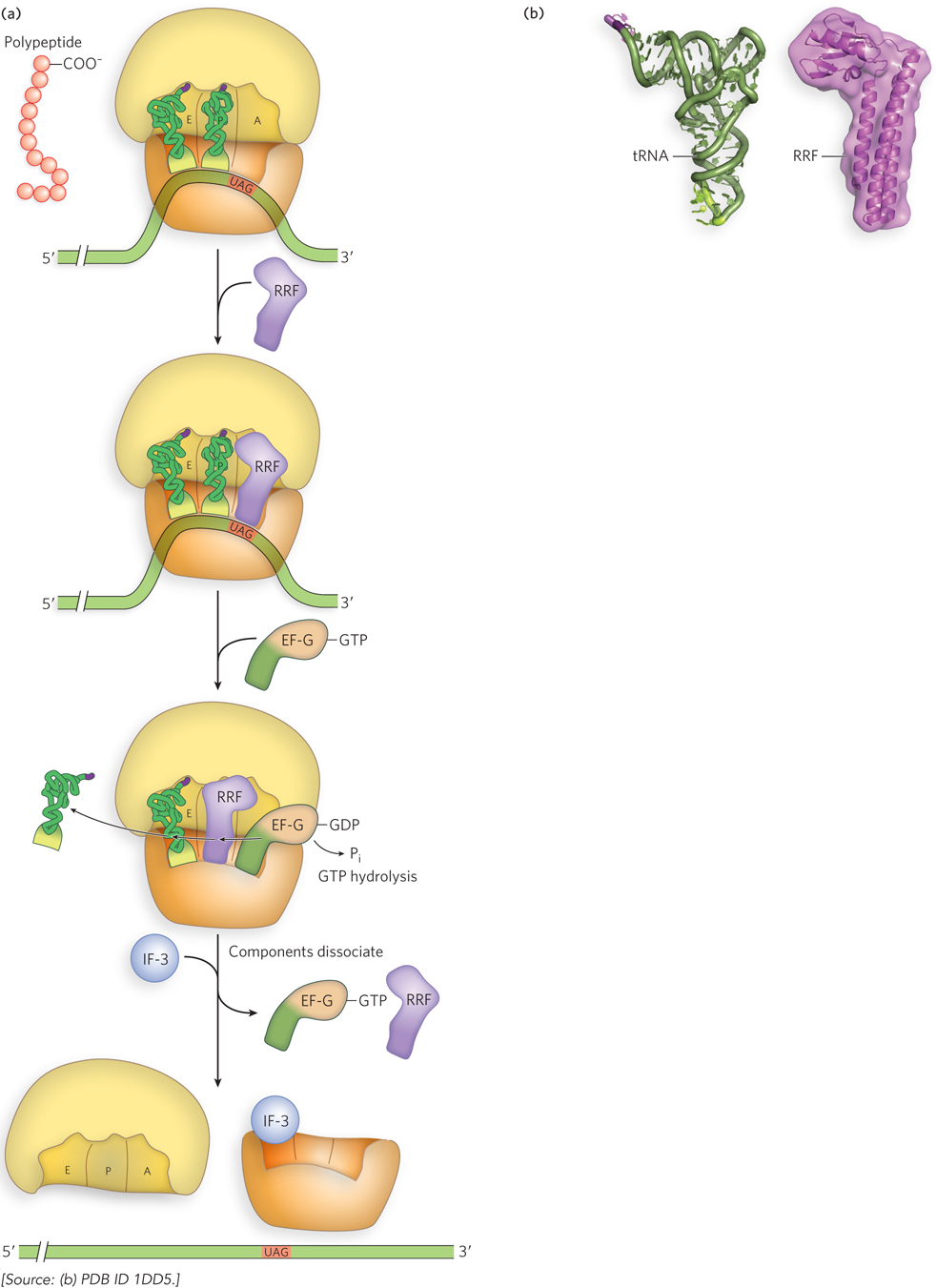
Figure 18-29: Ribosome recycling. (a) In bacteria, ribosome recycling factor (RRF) and EF-G separate the ribosome from the mRNA and tRNAs. (b) RRF is a protein with a three-dimensional shape very similar to that of a tRNA (shown here for comparison), allowing RRF to bind to the tRNA sites on the ribosome.
In a continuing story of molecular mimicry, RRF, like EF-G and EF-Tu–tRNA, fits into the ribosome’s tRNA-binding sites. GTP hydrolysis by EF-G is thought to result in translocation and displacement of the tRNAs, as occurs during translation. However, when RRF occupies the A site and translocates to the P site, the ribosome releases EF-G and RRF. The initiation factor IF-3 then binds the small ribosomal subunit and disaggregates the ribosome, thereby triggering release of the mRNA and preparing the small subunit for a new round of translation.
In eukaryotes, protein release factors (RFs) recognize the stop codon and stimulate hydrolysis of the ester bond linking the polypeptide chain with the peptidyl site tRNA, a reaction catalyzed by the ribosome’s peptidyl transferase center. There are two classes of RFs: class 1 RFs bind the stop codon directly, whereas class 2 RFs are not codon specific but serve to enhance class 1 RF activity and make the process GTP-dependent.
Fast and Accurate Protein Synthesis Requires Energy
The synthesis of a protein true to the information specified in the cell’s mRNA requires energy. Forming each aminoacyl-tRNA requires two high-energy phosphate groups. An additional ATP is consumed each time an incorrectly activated amino acid is hydrolyzed by the deacylation activity of an aminoacyl-tRNA synthetase, as part of its proofreading activity. One GTP is cleaved to GDP and Pi during the first elongation step, and another during the translocation step. Thus, on average, the energy derived from the hydrolysis of more than four NTPs to NDPs is required for the formation of each peptide bond.
This represents a large thermodynamic drive in the direction of synthesis: at least 4 × 30.5 kJ/mol = 122 kJ/mol of phosphodiester bond energy to generate a peptide bond, which has a standard free energy of formation of about 21 kJ/mol. The net free-energy change during peptide bond synthesis is thus −101 kJ/mol. Why would the cell need to expend so much energy on protein synthesis?
Proteins are information-containing polymers. The biochemical goal in the peptidyl transferase reaction is not simply the formation of a peptide bond but the formation of a peptide bond between two specified amino acids. Each of the high-energy phosphate compounds expended in this process plays a critical role in maintaining proper alignment between each new codon in the mRNA and its associated amino acid at the growing end of the polypeptide. This energy input permits very high fidelity in the biological translation of the genetic message of mRNA into the amino acid sequence of proteins.
Antibiotics and Toxins Frequently Target Protein Synthesis
Protein synthesis is the primary target of many naturally occurring antibiotics and toxins. Antibiotics are produced by bacteria or other microorganisms to inhibit protein synthesis in other bacteria—that is, these biochemical weapons are synthesized by some microorganisms and are extremely toxic to others. The differences between bacterial and eukaryotic protein synthesis, though often subtle, are sufficient that most (though by no means all) of the antibiotics discussed here are relatively harmless to eukaryotic cells. Because nearly every step in protein synthesis can be specifically inhibited by one antibiotic/toxin or another, antibiotics have become valuable tools in the study of protein synthesis.
Puromycin, made by the mold Streptomyces alboniger, is one of the best-understood inhibitory antibiotics. Its structure is very similar to the 3′ end of an aminoacyl-tRNA, so puromycin can bind to the ribosomal A site and participate in peptide bond formation, producing peptidyl-puromycin (Figure 18-30). However, because puromycin resembles only the 3′ end of the tRNA, it does not engage in translocation and dissociates from the ribosome shortly after it is linked to the C-terminus of the peptide. This prematurely stops protein synthesis.
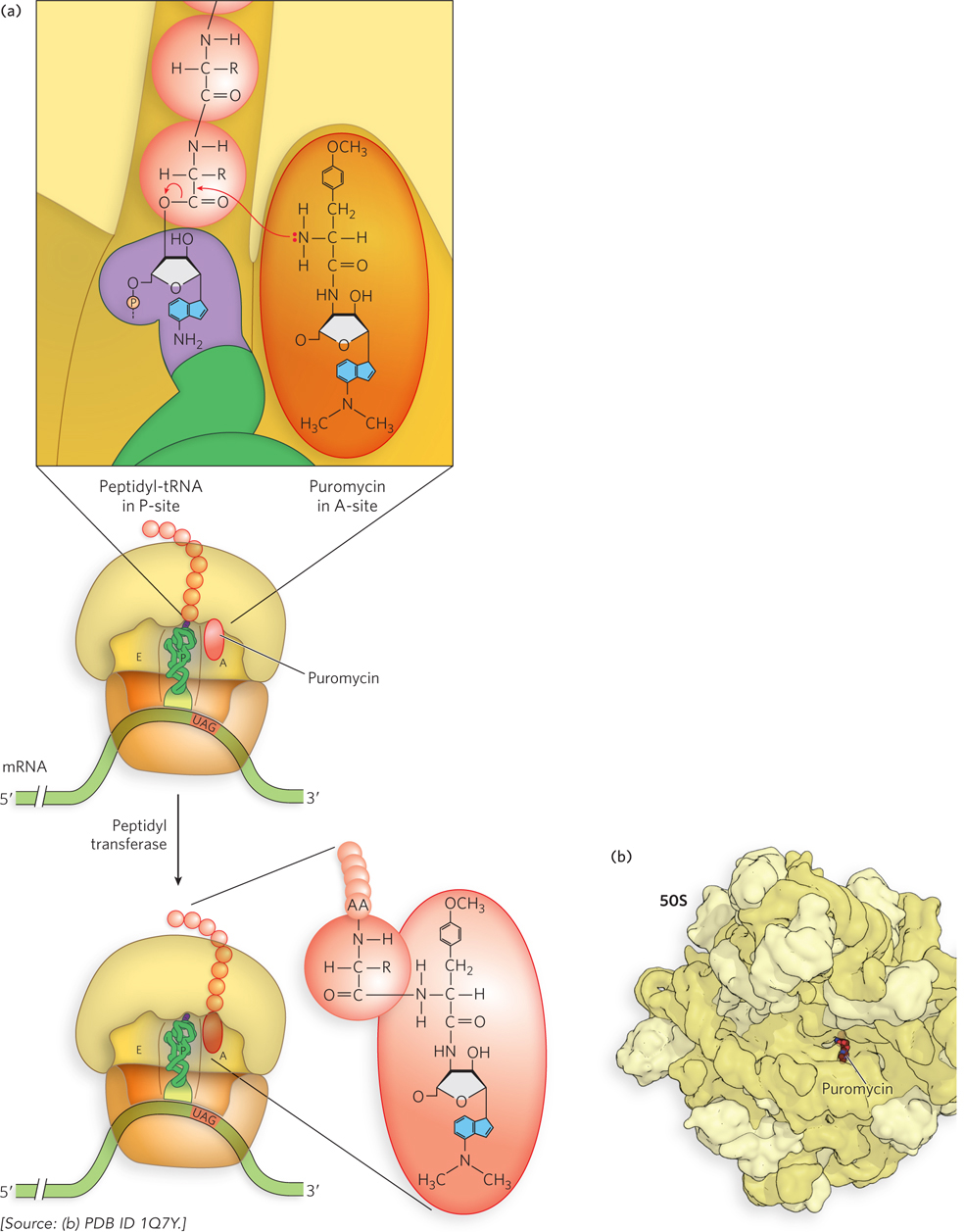
Figure 18-30: Puromycin as inhibitor of translation. (a) Puromycin inhibits translation by binding to the A site and mimicking the 3′ end of an aminoacyl-tRNA. The puromycin participates in the peptidyl transfer reaction, but because it is not bound to a tRNA, it is not anchored to the ribosome. (b) Crystal structure showing puromycin bound to the peptidyl transferase center of the bacterial 50S ribosomal subunit.
Tetracyclines inhibit bacterial protein synthesis by blocking the ribosomal A site, preventing the binding of aminoacyl-tRNAs. Chloramphenicol inhibits protein synthesis by bacterial (and mitochondrial and chloroplast) ribosomes by blocking peptidyl transfer; it does not affect cytosolic protein synthesis in eukaryotes. Conversely, cycloheximide blocks the peptidyl transferase of 80S eukaryotic ribosomes but not that of 70S bacterial (or mitochondrial and chloroplast) ribosomes. Streptomycin, a trisaccharide, causes misreading of the genetic code (in bacteria) at relatively low concentrations and inhibits initiation at higher concentrations.
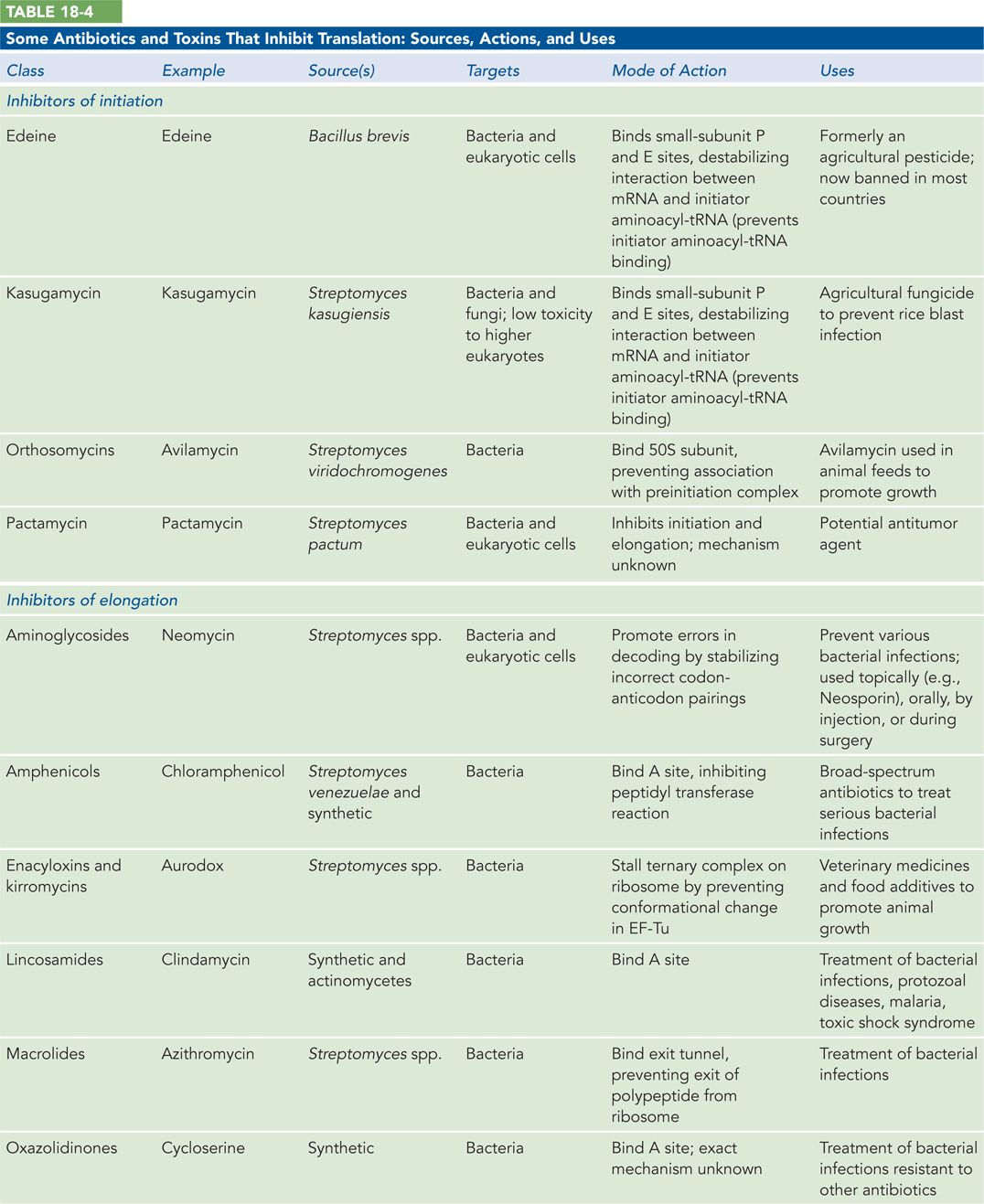
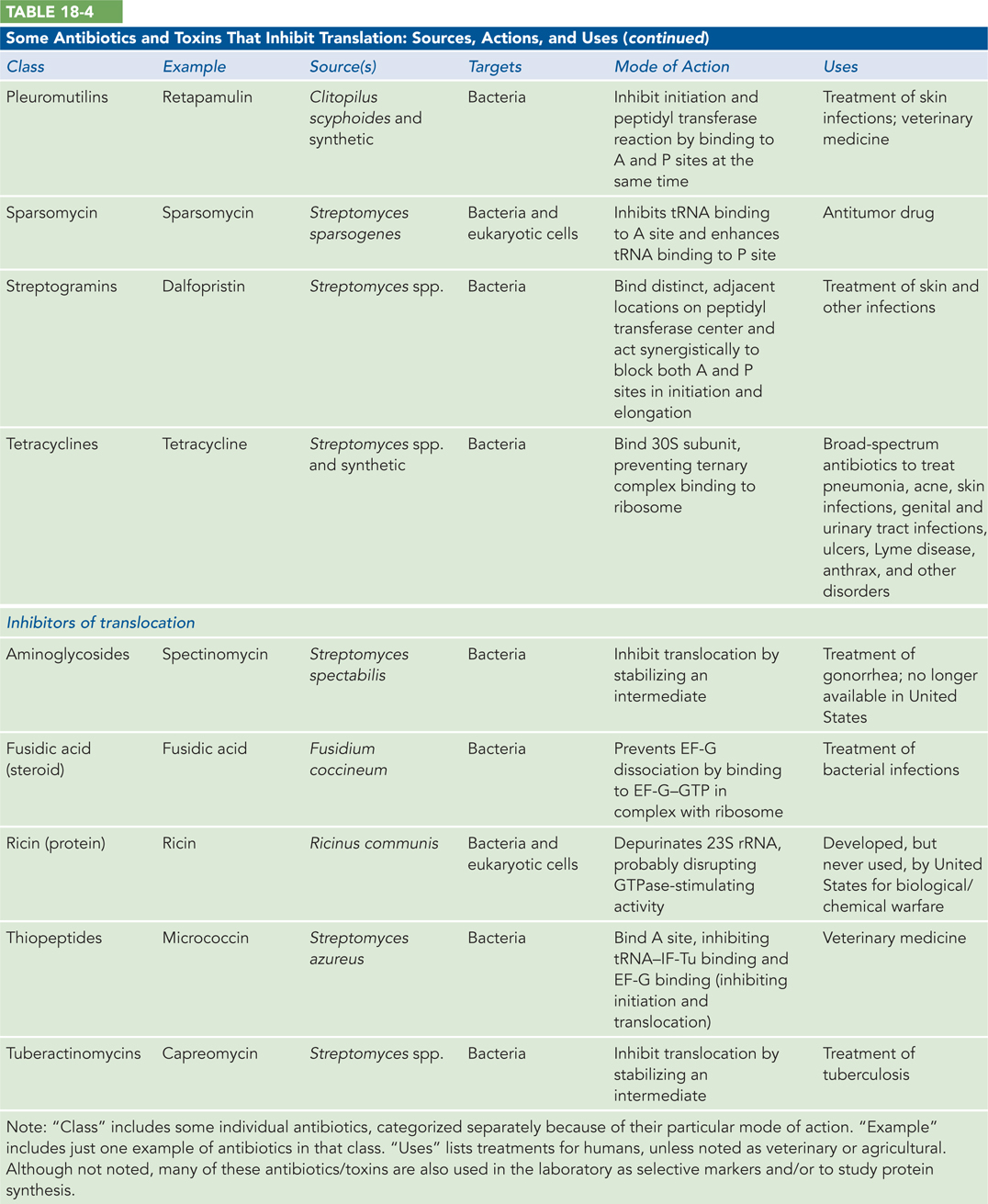
Several other inhibitors of protein synthesis are notable because of their toxicity to humans and other mammals. Diphtheria toxin is an enzyme that catalyzes the ADP-ribosylation of a diphthamide (a modified histidine) residue of the eukaryotic elongation factor eEF2, thereby inactivating it. The toxin is secreted by the bacterium Corynebacterium diphtheriae. Infected individuals experience fever, chills, neck swelling, and a fast heart rate. Although common historically, diphtheria has largely been eradicated in developed countries through widespread vaccination. Among the plant-produced toxins, ricin, an extremely toxic protein of the castor bean, inactivates the 60S subunit of eukaryotic ribosomes by depurinating a specific adenosine in 23S rRNA (Highlight 18-3). Table 18-4a and Table 18-4b list some antibiotics and toxins that target translation, their functional consequences, and some clinical and other uses.
HIGHLIGHT 18-3 MEDICINE: Toxins That Target the Ribosome
The toxic effects of two small proteins called ricin and abrin, derived from castor beans and jequirity peas, respectively, have been known and studied since ancient times. After a period of extensive study of these toxins at the end of the nineteenth century, the scientific community largely lost interest—until experiments published in the late 1960s and early 1970s showed that ricin and abrin inhibited protein synthesis in rats and cultured cells. The toxins did not interfere with the structure of polysomes, so researchers concluded that they acted on a component required for elongation of the polypeptide.
Studies showed that ricin and abrin required some time to exert their effects, suggesting that they might have enzymatic activity. Both toxins consist of two distinct, disulfide-linked polypeptide chains. The finding that reducing agents enhanced the toxins’ ability to inhibit protein synthesis in vitro suggested that the activity lies in one of the individual chains; in both toxins, this was found to be the smaller chain, termed the A chain. After cell fractionation to isolate ribosomes and smaller protein factors, experiments showed that the A chains act on the ribosomes. Further studies revealed that the target is the 60S ribosomal subunit.
In elegant studies in the 1980s, Ira Wool and Y. Endo found that the toxin A chain is a glycosidase (an enzyme that cleaves sugars at the glycosidic linkage) that removes the adenine from an A residue in an exposed loop of 28S rRNA (residue A4,324 in rat 28S RNA). Because this loop is involved in the binding of an elongation factor, the modified ribosomes are unable to support protein synthesis.
Ricin is legendary for its use as a murder weapon. In perhaps the most famous case, known as the Umbrella Murder, journalist Georgi Markov died as a result of an incident on a London street, reportedly killed by the Bulgarian secret police. Markov felt a pain like a bee sting on the back of his thigh. Turning, he saw a man pick up an umbrella from the ground and quickly get into a taxi. When Markov arrived at his office at the BBC World Service, the pain was increasing, and he noticed a red pimple forming at the site of the sting. Later that day he developed a fever and was admitted to a hospital, where he died a few days later. The cause of death was poisoning from a ricin-filled pellet, presumably fired from an umbrella tip. In a more recent example, ricin was used as a key plot device in the popular television series Breaking Bad.
SECTION 18.4 SUMMARY
In the first step of elongation in bacteria, the incoming aminoacyl-tRNA binds to the ribosome with the help of EF-Tu. This step requires GTP hydrolysis and allows time for proofreading of the codon-anticodon interaction. In the second step, a peptide bond is formed between the two amino acids bound by their tRNAs to the ribosomal A and P sites.
Peptide bond formation involves nucleophilic attack of the α-amino group of the A-site aminoacyl-tRNA on the carbonyl carbon of the ester bond linking the growing peptide chain to the P-site tRNA. This reaction is driven by a favorable change in entropy.
The third step of elongation is translocation of the peptidyl-tRNA from the A site to the P site. This is accomplished with the help of EF-G, a GTPase that is a structural analog of the EF-Tu–aminoacyl-tRNA complex.
The three steps of elongation are repeated for each codon in the mRNA; each cycle consumes two GTP molecules.
After many elongation cycles, release factors recognize the stop codon and terminate polypeptide synthesis by releasing the polypeptide, displacing the tRNAs, and separating the ribosomal subunits. In bacteria, ribosome recycling factor stimulates release of the tRNAs in the P and E sites, leading to release of the mRNA.
At least four high-energy phosphate bonds (from ATP and GTP) must be broken to generate each peptide bond, an energy investment necessary for guaranteeing the fidelity of translation.
Many well-studied and clinically important antibiotics and toxins inhibit some aspect of protein synthesis.










