18.5 TRANSLATION-COUPLED REMOVAL OF DEFECTIVE mRNA
Occasionally, mRNAs with premature stop codons (nonsense mutations, as described in Chapter 17) or with no stop codon at all (called non-stop mRNAs) arise from errors in DNA replication or transcription, or from mRNA degradation. Such defective mRNAs have the potential to produce nonfunctional or even toxic proteins, and they can also prevent efficient termination of translation and recycling of the protein synthesis machinery. For these reasons, elegant mechanisms have evolved to deal with such aberrant mRNAs during translation.
Ribosomes Stalled on Truncated mRNAs Are Rescued by tmRNA
Truncated, or non-stop, mRNAs occur when DNA transcription ends prematurely or when, due to a mutation, an mRNA lacks a stop codon. Truncated proteins produced by incomplete mRNAs, if inactive, could spell disaster for the cell, because they might contain the binding determinants that allow them to take the place of the active protein in a cellular activity. The ribosome takes care of this problem in a process that recognizes and removes the defective mRNA, as well as the defective protein.
When a ribosome reaches the 3′ end of a truncated mRNA, it stalls, unable to recruit either an appropriate tRNA or the proper release factors. In bacteria and some eukaryotic organelles, a fascinating quality-control pathway solves this problem with a 457 nucleotide RNA called tmRNA, also known as SsrA RNA or 10Sa RNA. A versatile, evolutionarily conserved bacterial molecule, tmRNA has the combined structural and functional properties of both a tRNA and an mRNA.
The 5′ terminus of tmRNA mimics the structure of tRNAAla and is charged with alanine by the Ala-tRNA synthetase. The alanine-charged tmRNA binds like a tRNA to the A site of the stalled ribosome, together with EF-Tu–GTP (Figure 18-31). The Ala-tmRNA donates its alanine to the nascent polypeptide chain in the P site, in a standard peptidyl transferase reaction. The tmRNA then takes the place of mRNA in the vacated mRNA channel of the ribosome stalled at the P site. The placement of another RNA molecule instead of an mRNA in this ribosome channel could not normally occur, because an intact mRNA would occupy the channel. But a prematurely terminated mRNA presents a vacant mRNA site for a tmRNA to occupy. Thus, tmRNA can act as a surrogate mRNA, replacing the truncated mRNA, with its self-encoded peptide reading frame directing synthesis of a 9 amino acid sequence before encountering a stop codon. Thus, counting the alanine, the tmRNA incorporates 10 amino acids at the C-terminus of the truncated protein.
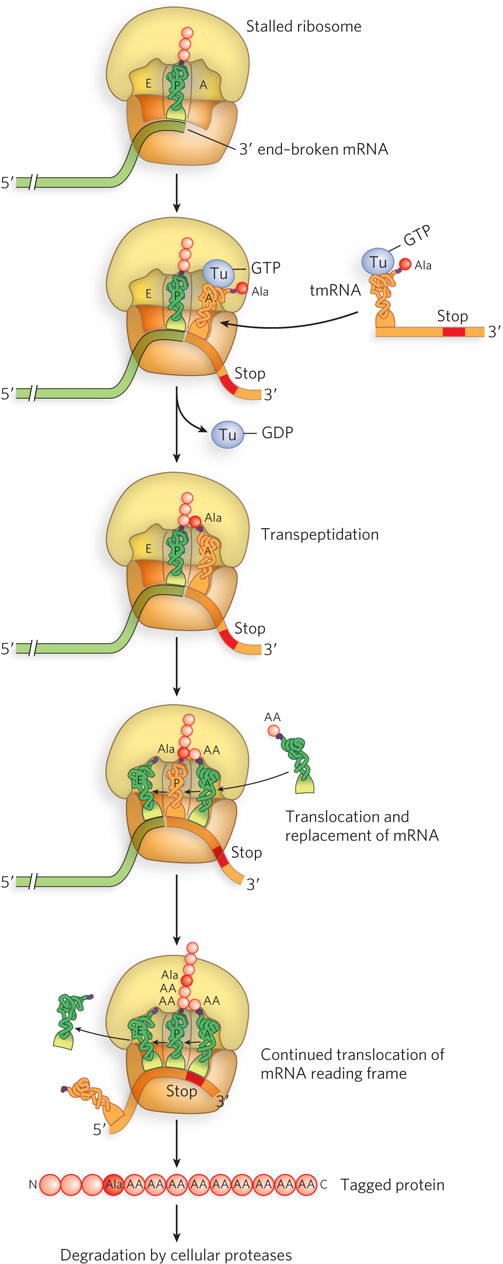
Figure 18-31: The rescue of stalled bacterial ribosomes by tmRNA. In bacteria, tmRNA rescues stalled ribosomes by mimicking both tRNA and mRNA, allowing release of the faulty mRNA while at the same time marking the truncated polypeptide for degradation. The encoded tag peptide (ANDENYALAA in E. coli) varies among different types of bacteria.
Translation terminates at the tmRNA stop codon, permitting disassembly and recycling of the ribosomal subunits. And in a remarkably strategic cellular maneuver, the 10 amino acid sequence encoded by tmRNA is a cellular signal that tags the protein for degradation—that is, the 10-residue degradation tag at the C-terminus is recognized by C-terminal–specific cellular proteases. The proteases denature and degrade tagged proteins in both the cytoplasm and the periplasmic space (between the inner plasma membrane and outer membrane of some bacteria). Furthermore, in addition to ribosome rescue and protein tagging, the tmRNA salvage system facilitates degradation of the defective mRNA by the enzyme RNase R.
Although not essential in E. coli, tmRNA activity is required for bacterial survival under adverse conditions and for virulence in some (perhaps all) pathogenic bacteria. Recent evidence suggests that in addition to its quality-control function, the tmRNA system might play a key role in regulating proteins for which cellular concentrations are particularly sensitive to the balance between proteolysis and the cell’s translational efficiency.
Eukaryotes Have Other Mechanisms to Detect Defective mRNAs
Eukaryotes respond to non-stop mRNAs in other ways, not using the tmRNA mechanism described above. Because eukaryotic mRNAs contain a 3′ poly(A) tail, an mRNA without a stop codon is translated through the tail to produce a string of Lys residues at the C-terminus of the polypeptide (AAA encodes lysine). The ribosome stalled at the end of the non-stop mRNA binds to the protein Ski7 (related to eRF3), which initiates non-stop mRNA decay (Figure 18-32). Ski7 triggers dissociation of the ribosome and degradation of the non-stop mRNA by recruiting the exosome ribonuclease that cleaves in the 3′→5′ direction (see Figure 16-25). The defective polypeptide is rapidly degraded by a protease that recognizes the C-terminal poly(Lys) tag.
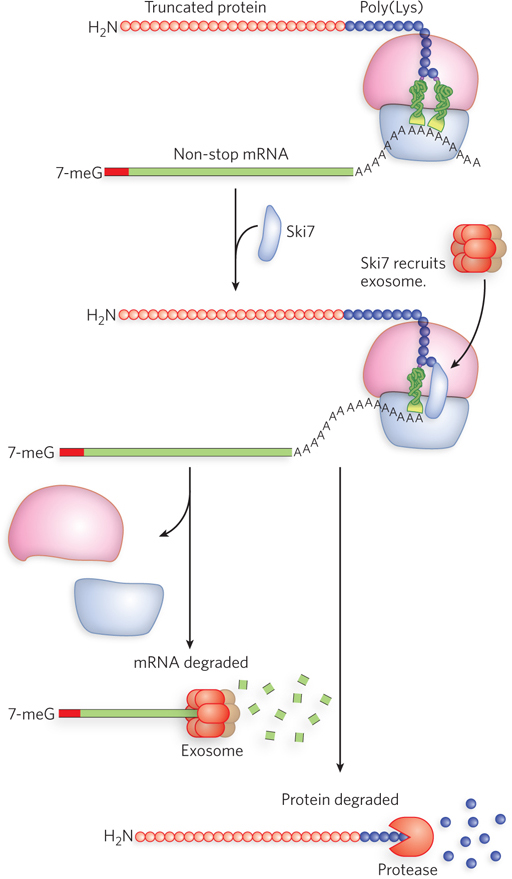
Figure 18-32: The rescue of stalled eukaryotic ribosomes by non-stop mRNA decay. Ribosomes continue to translate through the poly(A) tail of a non-stop mRNA, resulting in a poly(Lys) protein tail. Ski7 binds to the ribosome and recruits an exosome to degrade the non-stop mRNA and a protease to degrade the protein.
A process known as nonsense-mediated mRNA decay has evolved in eukaryotes to detect and destroy mRNAs that contain a premature stop, or nonsense, codon (Figure 18-33). This process works through the splicing machinery in the nucleus, before the mRNA is exported to the cytoplasm for translation. During pre-mRNA splicing, the site of each excised intron is marked by the presence of an exon junction complex (EJC), positioned on the 5′ side of the exon-exon boundary (see Chapter 16). During the first round of translation, the ribosome removes the EJCs as it moves along the mRNA from the start to the end of the coding region. If a premature stop codon is encountered, however, the ribosome is released early, before all the EJCs have been removed. In this event, Upf1 and Upf2 proteins are recruited by the leftover EJC(s), which in turn recruit an enzyme that cleaves the 5′ cap from the mRNA. Without the cap to protect it, the end of the RNA is highly susceptible to degradation by an exoribonuclease that hydrolyzes RNA from the free 5′ end.
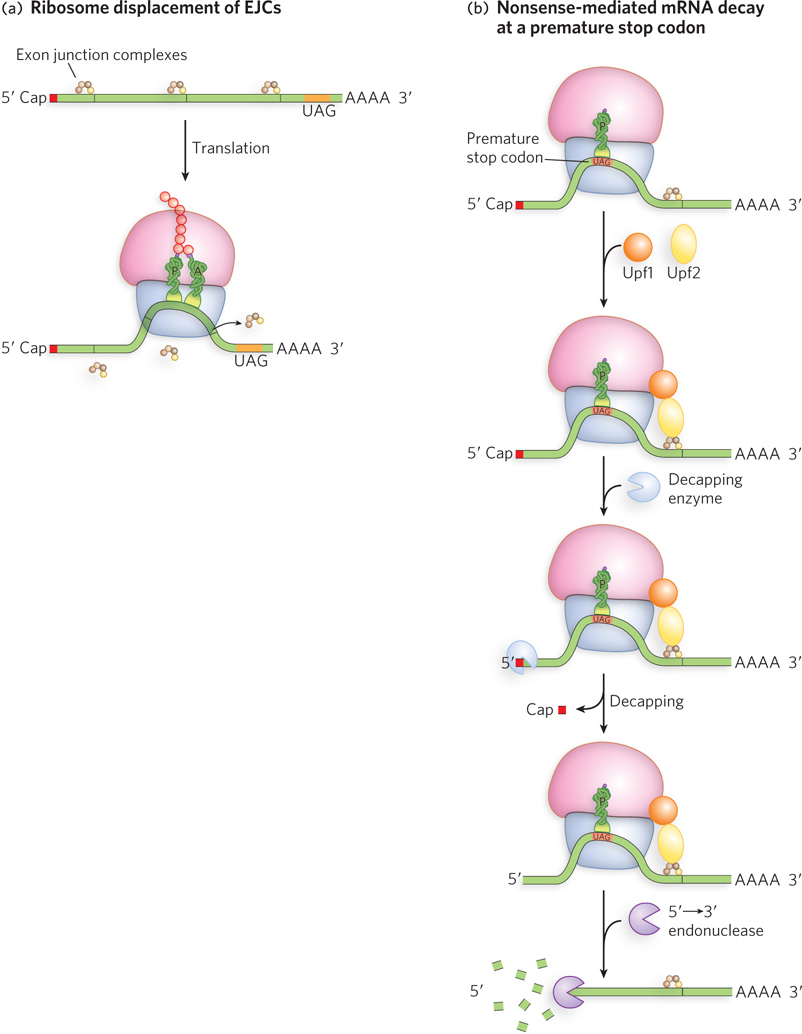
Figure 18-33: Eukaryotic nonsense-mediated mRNA decay. (a) The spliceosome deposits exon junction complexes (EJCs) at the splice sites of an mRNA (see Chapter 16), and the EJCs are usually displaced by the ribosome as it travels along the mRNA. (b) If the ribosome encounters a premature stop codon upstream of an EJC, then Upf1 and Upf2 recruit a decapping enzyme and a 5′→3′ exonuclease to promote degradation of the mRNA.
Note that both of the above processes for removing damaged or truncated mRNA are performed by the ribosome, and therefore the ribosome is an effective proofreader. The recognition of defective mRNAs thus requires active translation. Recent evidence suggests that such mechanisms play significant roles in the regulation and evolution of protein function, as we will discuss in detail in Chapter 22.
SECTION 18.5 SUMMARY
In bacteria, tmRNA rescues ribosomes stalled on non-stop mRNAs, playing the role of both tRNA and mRNA in the A site. Translation continues, using the tmRNA as template and ending at the tmRNA stop codon.
Eukaryotic non-stop mRNAs are translated through the poly(A) tail, encoding a string of Lys residues that tag the protein for degradation.
In eukaryotes, pre-mRNA splicing leaves an exon junction complex on spliced mRNAs. If a ribosome encounters a stop codon before it has removed all the EJCs, the stop codon is identified as premature, and the mRNA is tagged for degradation.


