22.2 TRANSLATIONAL CONTROL IN THE CYTOPLASM
Regulation at the level of translation assumes a much more prominent role in eukaryotes than in bacteria and is observed in a range of cellular situations. In contrast to the tight coupling of transcription and translation in bacteria, transcripts generated in a eukaryotic nucleus must be processed and transported to the cytoplasm before translation. This can impose a significant delay on the availability of a protein function. When a rapid increase in protein production is needed, a translationally repressed mRNA already in the cytoplasm can be activated for translation without delay.
Translational control is responsible for activating the expression of proteins necessary for cell fate decisions. Translational regulation may play an especially important role in controlling the expression of certain very long eukaryotic genes (a few are measured in millions of base pairs!), for which transcription and mRNA processing can require many hours. Some genes are regulated at both the transcriptional and translational stages, with the latter playing a role in fine-
Eukaryotes have at least three main mechanisms for regulating translation. First, various initiation factors are subject to phosphorylation by protein kinases. The phosphorylated forms are often less active and generally depress translation in the cell. Second, some proteins bind directly to mRNA and act as translational repressors. Many bind at specific sites in the 3′UTR and interact with other initiation factors bound to the mRNA, or they interact with the 40S ribosomal subunit to prevent initiation. Third, binding proteins can disrupt the interaction between eIF4E and eIF4G (recall from Chapter 18 that interaction between these initiation factors is required for proper assembly of the ribosome-
766
The variety of translational regulation mechanisms in eukaryotes provides flexibility, allowing focused repression of a few mRNAs or global regulation of all cellular translation. Here we explore several examples in which these mechanisms come into play.
Initiation Can Be Suppressed by Phosphorylation of eIF2
Translation initiation in eukaryotes is a complex process involving multiple initiation factors that recruit ribosomes to mRNAs. Reversible phosphorylation of initiation factors plays a central role in regulating initiation. The phosphorylation is triggered by a wide range of cellular conditions, depending on cell type and function. One of the main phosphorylation pathways involves eIF2. In all eukaryotes, eIF2 is composed of three polypeptide subunits—
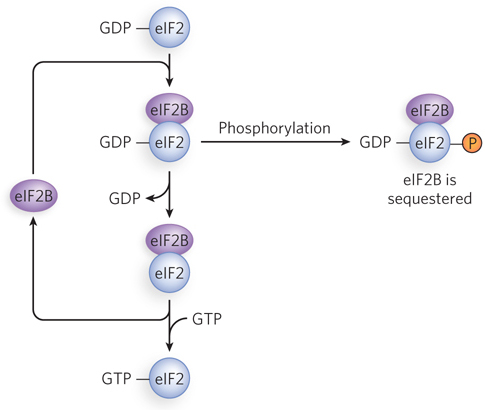
This process has been well studied in mammalian reticulocytes. The maturation of reticulocytes into red blood cells includes destruction of the cell nucleus, leaving behind a hemoglobin-
Phosphorylation of eIF2α regulates translation in other systems as well. For example, a double-
The 3′UTR of Some mRNAs Controls Translational Efficiency
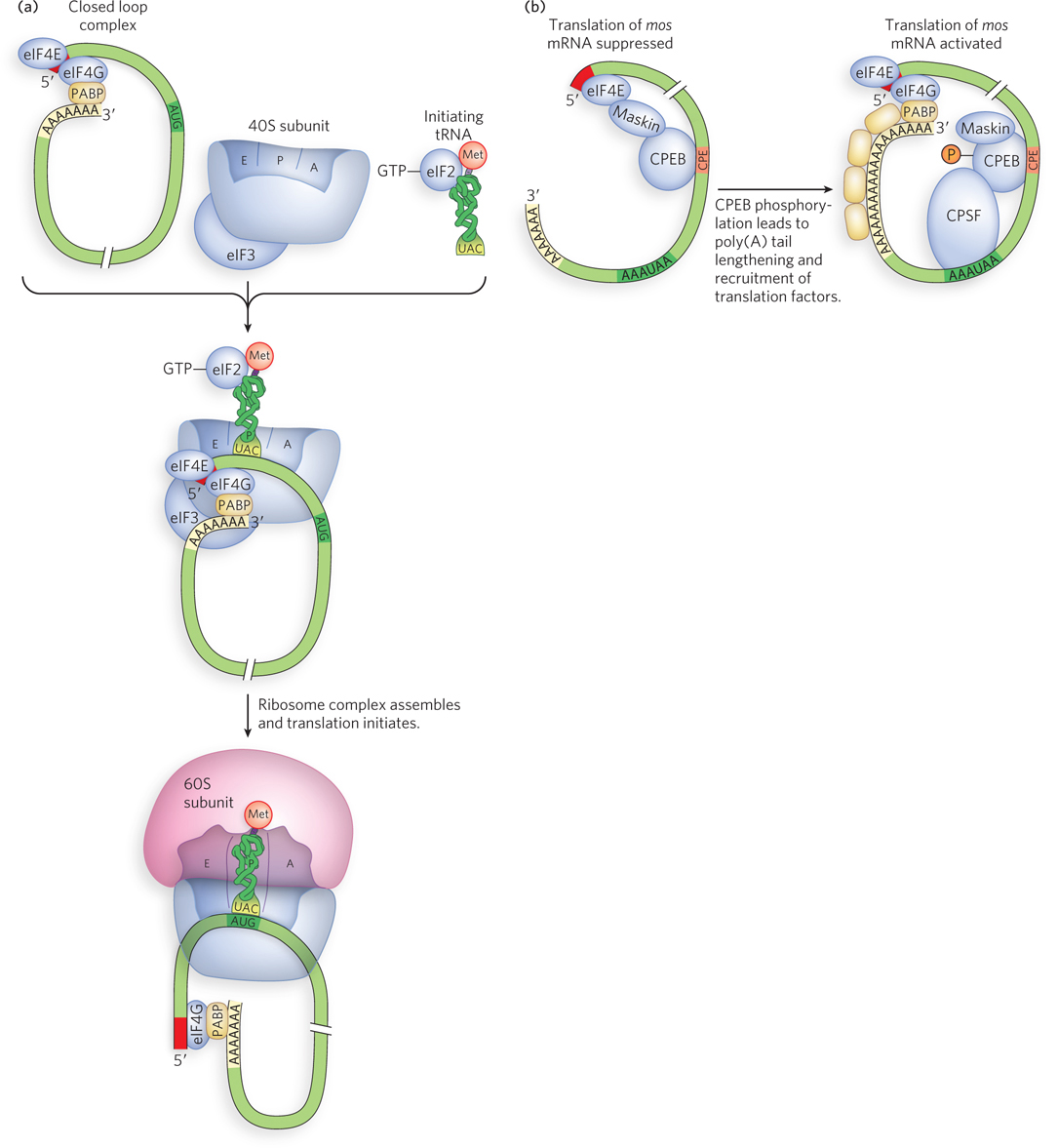
The 3′ untranslated region of an mRNA communicates with the 5′ end through protein-
767
Regulation of translation by protein binding to the 3′UTR is especially important during the development of early embryos. Many maternal RNAs (deposited in the egg cytoplasm during oogenesis) have relatively short poly(A) tails (20 to 40 A residues), and their translation is suppressed until it is needed. Activation requires two sequences in the 3′UTR: the nuclear AAUAAA polyadenylation sequence and the cytoplasmic polyadenylation element (CPE, with consensus sequence UUUUUAUU). CPEs are bound by the protein CPEB (CPE-
768
In addition to CPEBs, other types of proteins bind the 3′UTR of mRNA to regulate translation, including proteins of the PUF family (named for Pumilio and FBF, the first two proteins of this type to be discovered). PUF proteins are a highly conserved family of RNA-
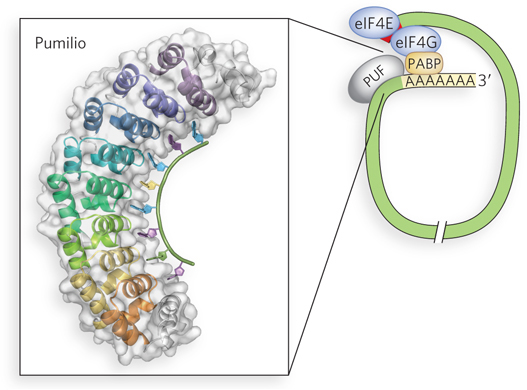
Each PUF protein is thought to regulate multiple mRNAs, because experiments have demonstrated the proteins’ ability to bind multiple targets. Researchers have engineered fruit flies to express a molecularly tagged version of Pumilio. The tagged protein was purified with its bound RNA partners, and the identities of the bound RNAs were determined using microarray technology (see Chapter 7). The study showed that many of the bound mRNAs shared a short, characteristic sequence in their 3′UTRs. Furthermore, the mRNAs tended to encode functionally related proteins, suggesting that Pumilio acts by binding to sets of mRNAs. Many of the proteins regulated by Pumilio function in developmental pathways considered in Section 22.5.
Once bound by the PUF protein, the targeted mRNAs are typically blocked from efficient translation on the ribosome. Although the mechanism of repression is not completely known, PUF proteins seem to block initiation. In some cases, PUF proteins may also increase the rate of mRNA degradation.
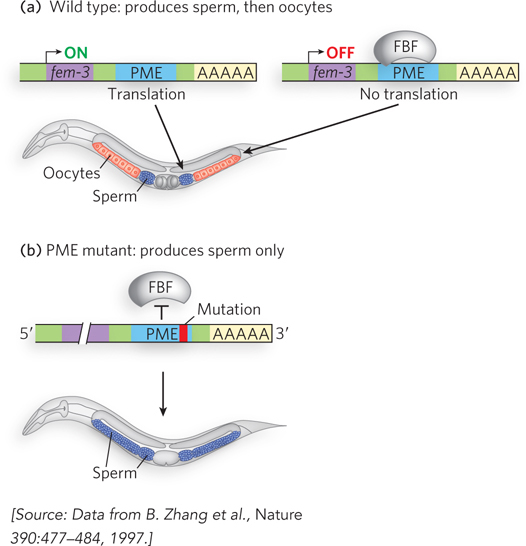
In the nematode Caenorhabditis elegans, the hermaphrodite produces sperm and oocytes, successively, during development of the germ line. This cell fate decision is controlled by the PUF family protein FBF (fem-
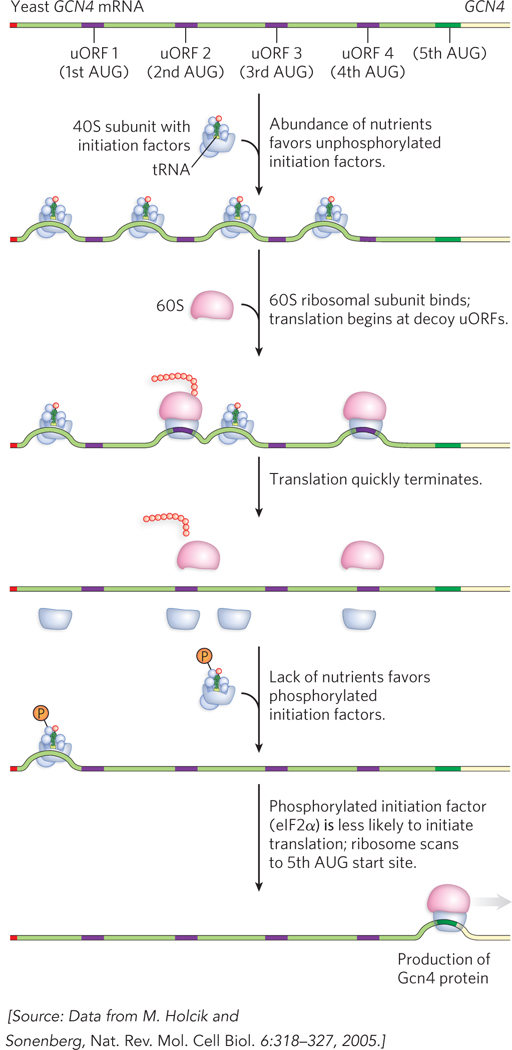
Upstream Open Reading Frames Control the Translation of GCN4 mRNA
Some eukaryotic genes are controlled by short open reading frames located upstream from the gene’s authentic start codon. These upstream open reading frames (uORFs) do not produce functional protein. Instead, they are a gene regulatory mechanism that generally decreases translation by diverting ribosomes, often making them halt and dissociate before reaching the AUG start codon. It might seem that uORFs, rather than regulating levels of gene expression, would simply repress genes under all conditions. However, the effectiveness of uORFs in terminating ribosome activity is altered by phosphorylation of eIF2α. An instance of this type of regulation is observed for the yeast GCN4 gene (general control nonderepressible), encoding a transcription activator that regulates many other genes.
Although the phosphorylation of eIF2α typically down-
769
The mechanism of activation involves four short uORFs preceding the Gcn4-
mRNA Degradation Rates Can Control Translational Efficiency
Another important way in which translation is regulated in eukaryotic cells is by the degradation of mRNAs. Translation and degradation of particular mRNAs often show an inverse relationship. Those mRNAs that are efficiently translated tend to be stable in the cytoplasm, whereas those that are poorly translated tend to be degraded more quickly. The discovery of specific pathways of mRNA degradation has led to a better understanding of the close correlation between mRNA translation and turnover. Decay of mRNAs begins with removal of the 3′ poly(A) tail (deadenylation), followed by removal of the 5′ 7-
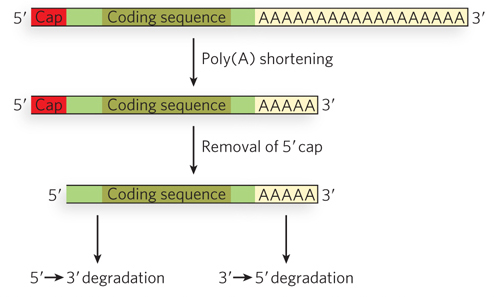
770
Removal of the 5′ cap involves the formation of macromolecular assemblies of translationally repressed mRNAs bound to decapping enzymes. Known as processing bodies (P bodies) (see Figure 16-26), these large cytoplasmic structures are easily seen by light microscopy in cells that have been starved or otherwise stressed to induce general translational repression.
Studies of the composition and formation of P bodies suggest that the rates of mRNA translation and degradation are influenced by the relative stability of the mRNA bound in polyribosomes and in P bodies. Regulatory proteins that bind sequences or structures within related groups of mRNAs serve either to recruit those mRNAs to P bodies or to stabilize their interaction with ribosomes. This is an exciting area of active research. P bodies are part of the conserved translational control machinery in eukaryotic cells, influencing patterns of gene expression in cells as diverse as oocytes and neurons.
SECTION 22.2 SUMMARY
Eukaryotes have at least three mechanisms of translational regulation: phosphorylation-
dependent repression of initiation factors, direct binding of mRNAs by translational repressors, and disruption of the required interaction between eIF4E and eIF4G. Reversible phosphorylation of initiation factors plays a central role in regulating translation initiation in response to cellular conditions. When the eIF2α subunit is phosphorylated, eIF2 forms a stable complex with eIF2B, making these factors unavailable for further rounds of translation initiation.
The CPE-
binding protein binds sequences in the 3′UTR of maternal mRNAs and contributes both to suppressing translation (in conjunction with Maskin, a 4E- BP- like protein) and to activating translation (through interaction with CPSF). All PUF proteins have characteristic sequence and structural features that enable their binding to the 3′UTRs of mRNAs. Binding leads to translational repression and/or degradation of the transcript, reducing expression levels.
In yeast cells, upstream open reading frames (uORFs) in the GCN4 mRNA, preceding the Gcn4-
coding sequence, act as ribosome decoys. They prevent ribosomes from initiating translation at the GCN4 start site when nutrients are abundant, allowing ribosomes to initiate efficiently at the uORFs instead of at the authentic start site farther downstream. Efficiently translated mRNAs tend to be stable in the cytoplasm; those that are poorly translated tend to be rapidly degraded. The decay of an mRNA involves removal of the 3′ poly(A) tail, decapping of the 5′ end, then exonuclease-
catalyzed degradation. Cytoplasmic foci, known as P bodies in yeast, are sites of mRNA decapping and degradation.