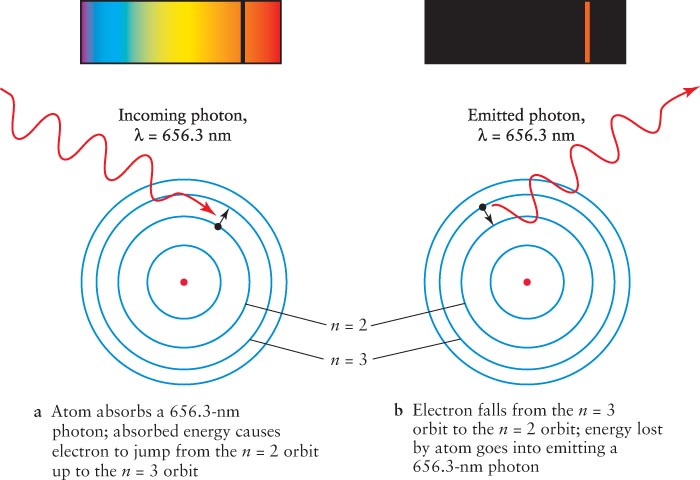
Figure 3-