Chapter 1. Molecules and Compounds
Introduction
“There are the rushing waves… mountains of molecules, each stupidly minding its own business… trillions apart …yet forming white surf in unison.”
Richard Feynman (1918 – 1988) American physicist who received the Nobel Prize in Physics in 1965 for his work on quantum electrodynamics and the physics of elementary particles.
The entire world that we see and know is made of chemical compounds and molecules. In addition to the chemicals of the natural world – in the earth’s crust, the oceans, and the atmosphere – a large number of other compounds and molecules are produced in and by living things. Human activities, for good or ill, have produced many more. Astonishingly, more than 50 million different chemicals have been identified and catalogued! Even more remarkably, just a few moments after the Big Bang, the universe consisted of nothing more than hydrogen atoms, and a small amount of helium. Chemists have devised a set of simple rules for naming and describing this vast array of compounds and molecules, and these are the subject of this chapter. We begin, however, with another look at the Periodic Table of the Elements, which will set the stage for our discussion of compounds and molecules.
1.1 What Relationships are Revealed in the Periodic Table of the Elements?
A total of 118 chemical elements have been discovered so far, either found naturally on the Earth or synthesized. It is likely that other elements will be discovered in the future as well. As we learned in Chapter 2, all the atoms of a given element have the same number of protons, and that number is referred to as the atomic number of that element. The atomic numbers of the chemical elements range from 1 to 118, and every number in this range represents one of the known elements. Hydrogen, with a single proton, has an atomic number of 1, whereas carbon, with six protons, has an atomic number of 6, and uranium, with an atomic number of 92, has 92 protons in its nucleus.
Each of the names of the chemical elements tells a story – describing for example the properties of the element, the place where it was discovered, an association with a mythological figure or a planet, or a famous chemist (Table 3.1). Each element is also represented by a unique chemical symbol of one or two letters. When the names of two or more elements begin with the same letter, all but one of them have a two-letter symbol, and the second letter is always lower case. Thus N stands for nitrogen, whereas Ni is for nickel, Nb is for niobium, Nd is for neodymium, and No is for nobelium. Most of the chemical symbols derive from the name of the element. Thus the symbol for hydrogen is H, O is for oxygen, Ca is for calcium, Si represents silicon, phosphorus is P, and bromine is Br. However, eleven of the elements have chemical symbols that do not correspond to their names (Table 3.2). These are all elements known since antiquity, and for these the symbols are derived from their earliest names, many of which were Latin.
By the middle of the 19th century, chemists were beginning to appreciate that groups of the sixty or so known elements shared similar properties. For example, lithium (atomic number 3), sodium (11), and potassium (19) share similar properties, as do beryllium (4), magnesium (12), and calcium (20) (Figure 1.11).

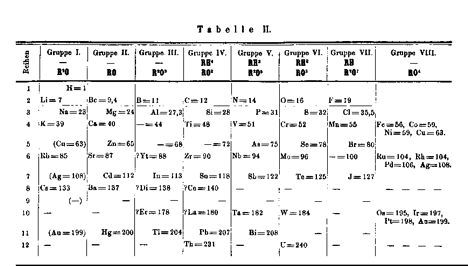
At least a half-dozen chemists provided early clues to these periodic relationships, but the work of a Russian chemist, Dmitri Mendeleev, was most influential. On a winter day in 1869, Mendeleev wrote out separate cards for the 63 elements known at that time. On each card, he noted the chemical and physical properties of the element, as well as its atomic weight. Mendeleev spent several hours moving the cards around on a table into different arrangements, comparing atomic weight and chemical and physical properties, until he had produced a chart with columns of chemically similar elements arranged in order of atomic weight (Figure 3.2).

Mendeleev’s insights led him to formulate his periodic law:
The physical and chemical properties of elements are periodic functions of their atomic mass.
The most compelling evidence that Mendeleev’s periodic table was valid arose from his ability to predict the existence of elements that fit into his periodic relationships but which had not yet been discovered. He predicted that new elements should fall in his table below boron, aluminum, and silicon. He even predicted their atomic weights, densities, and melting points. He called them “eka-boron”, “eka-aluminum”, and “eka-silicon”, respectively (from the Sanskrit eka- for “one”, to indicate that these elements lay one place below boron, aluminum, and silicon). Eka-boron was discovered in 1879 in Sweden by Lars Nilson, who named it scandium, for Scandinavia. Eka-aluminum was discovered in 1875 by Paul-Emile Lecoq de Boisbaudran, who named it gallium, after the Latin name for his native France. And eka-silicon was discovered in 1886 by Clemens Winkler in Freiberg, Germany. Winkler named it germanium, from the Latin name for Germany. Remarkably, the atomic weights, densities, and melting points of all three of these turned out to be almost exactly what Mendeleev had predicted!
These periodic relationships among the elements are summarized in the modern periodic table of the elements (Figure 3.3).
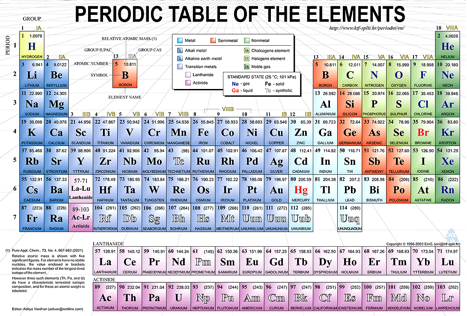
The elements in the periodic table are arranged in eighteen vertical columns called groups and seven horizontal rows called periods. Period 1 contains only hydrogen and helium, Periods 2 and 3 contain eight elements each, Periods 4 and 5 contain eighteen elements each, and Periods 6 and 7 contain 32 elements each. (In order to fit the table conveniently on the page, fourteen elements of Period 6 and fourteen elements of Period 7 are shown in separate rows at the bottom of the table. The lanthanides (atomic numbers 58-71) fit between lanthanum (57) and hafnium (72), whereas the actinides (atomic numbers 90-103) fit between actinium (89) and rutherfordium (104).
Groups 1A through 8A are known as the main groups and their elements are termed the main group elements, Groups 1B through 8B are the transition metal groups, and these elements are termed the transition elements. The lanthanides and actinides are known collectively as the inner transition metal groups.
Elements in each group of the table share similar chemical and physical properties, and many of the groups have names that emphasize these shared properties (Table 3.3). In Chapters 6 and 7, we will learn that the arrangements of electrons in the elements accounts exquisitely for these shared properties. It is remarkable that Mendeleev and others were able to describe the periodic properties among the elements fifty years before the electron was discovered, and sixty years before chemists had begun to understand the structures of atoms in any detail!
The elements on the left side and middle of the periodic table (excepting hydrogen) are termed metals or metallic elements (Figure 3.4).
They are typically lustrous substances, they conduct heat and electricity effectively, and they tend to lose electrons in chemical reactions. With the exception of mercury, they are solids at room temperature. Hydrogen and the elements on the upper right of the periodic table are called nonmetals. They are more varied in their physical properties, they are poor conductors of heat and electricity, and they typically gain electrons in chemical reactions. Metals and nonmetals are separated in the periodic table by a diagonal, zigzag line running from boron (B) to astatine (At). The elements lying on either side of this line, including boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and astatine (At), are called metalloids. Their properties are intermediate between those of the metals and nonmetals. Several of the metalloids (for example, silicon, germanium, antimony, and arsenic) are called semiconductors because they display electrical conductivity between that of the metals and non-metals. Semiconductor materials are the basis of all modern electronic devices, including cell phones, computers, digital televisions, and communication satellites.
Solved Problem 3-1:
(a) Give the chemical symbols for neon, potassium, copper, arsenic, strontium, iridium, dysprosium, and curium.
Solution: Ne, K, Cu, As, Sr, Ir, Dy, and Cm
(b) Name the elements represented by the symbols He, Sc, Ni, Se, Zr, Fr, Pa, Pu, and Es.
Solution: helium, scandium, nickel, selenium, zirconium, francium, protactinium, plutonium, and einsteinium.
Why could the symbols Naa, DD, and Pm not be used for new elements that might be discovered in the future?
Solution: Naa could not be used, because element symbols must be either one or two letters. DD would be inappropriate because it is customary to capitalize only the first letter in an elemental symbol. Pm could not be used, because it is already used for the element promethium (atomic number 61).
1.2 What is a Molecule?

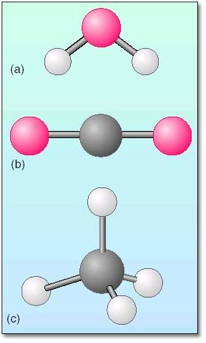
Most of the air that we breathe is nitrogen (N2) and oxygen (O2). Three other elements – sulfur, phosphorus, and selenium – exist as polyatomic molecules at room temperature. Whereas some molecules consist of several atoms of the same element, most molecules consist of two or more atoms of different elements (Figure 3.6).
A molecule is a combination of two or more atoms held together in a particular shape or structure by attractive forces. The smallest molecules consist of only two atoms. These are termed diatomic molecules. Large molecules may contain thousands of atoms. Seven of the chemical elements – including hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine – exist as diatomic molecules at room temperature (Figure 3.5).
Solved Problem 3-2: (a) In addition to diatomic oxygen (O2), which we breathe, there is another less common molecular form of oxygen. Do an internet search to discover what this form is, how it is formed in both the upper and lower atmospheres, and the health effects of each.
Solution: Ozone (O3) is a triatomic molecular form of oxygen. It is formed in the stratosphere (10-50 km, or 6-30 miles) above the earth, by the action of ultraviolet rays from the sun on O2. Here it constitutes “the ozone layer”, which protects us from harmful UV rays of the sun. In the lower atmosphere – called the troposphere – ozone is produced by the action of sunlight on emissions from cars and other fossil-fuel-burning sources. Here it is a harmful pollutant that can cause lung damage and respiratory problems.
1.3 What are Ions?
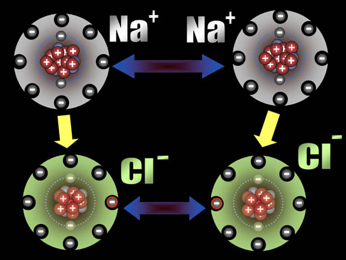
An atom is electrically neutral (with zero net charge) when it has an equal number of protons and electrons. That is, the negative charge from the electrons is exactly equal to or balanced by the positive charge of the protons in the nucleus. However, neutral atoms can either lose or gain electrons to become electrically charged particles called ions. For example, consider a neutral sodium atom, with 11 protons and 11 electrons. If this neutral atom loses an electron, the result is a positively charged particle, Na+. A positively charged atom or molecule is called a cation (pronounced cat’-eye-on):
Na → Na+ + e-
(Add an unnumbered figure here showing sodium atom and sodium ion with numbers of protons and electrons indicated.)
The charge on an ion is indicated by a right superscript quantity – in Eqn. 3-1, a “+” sign. When a neutral chlorine atom gains an electron, the result is a negatively charged particle with 17 protons and 18 electrons. A negatively charged atom or molecule is termed an anion (pronounced an’-eye-on):
Cl + e- → Cl-
Whenever an ion forms, an electron must be transferred from one atom to another. Electrons, like all matter and energy, are conserved, and thus are neither created nor destroyed.
Many atoms have a tendency to gain or lose electrons to end up with the same number of electrons as the noble gas that is closest in the periodic table. Recall from Chapter 1 that the noble gas elements are very stable and chemically non-reactive. This stability arises from the particular arrangement of electrons in each of the noble gases.. For example, loss of an electron by a sodium (Na) atom leaves 10 electrons, the same as contained in neon (Ne). Similarly, when a chlorine (Cl) atom gains an electron, it has a total of 18 electrons, the same as argon (Ar). This simple concept explains the formation of many ions, as shown in Figure 3.7.
Metals tend to lose one or more electrons to form cations, whereas nonmetals typically gain one or more electrons to form anions. The alkali metals (Li, Na, K, Rb, Cs) readily lose one electron to end up with the same number of electrons as the noble gas elements just preceding them in the periodic table. Alkaline earth metals (Mg, Ca, Sr, Ba) typically lose two electrons for the same reason. On the other hand, the halogen elements (F, Cl, Br, I) tend to form ions by gaining one electron, and the Group 6A elements (O, S, Se, Te) typically gain two electrons to form stable ions. Nitrogen, in Group 5A, forms the N3- ion by gaining three electrons (Figure 3.8).
Solved Problem 3-3: Write equations to show ion formation by Rb, Mg, I, and Te.
Solution: Rubidium, Rb, is in Group 1A of the periodic table and like other alkali metals tends to lose one electron to form a cation: Rb → Rb+ + e-
Mg → Mg2+ + 2e-
I + e- → I-
Te + 2e- → Te2-
1.4 What is a Compound?
Very few of the known elements exist in the free state on earth. Most elements are found in various compounds. A compound is a substance made up of atoms of two or more elements, combined in a fixed ratio. Nitrous oxide (N2O), also known as “laughing gas”, is a compound that always occurs in the ratio of two nitrogen atoms to one oxygen atom. In mixtures, on the other hand, elements can be combined in any ratio. (For example, nitrogen gas and oxygen gas can be combined to form mixtures of any desired proportions.) Note that there is an important distinction between the definition of a compound here and the earlier definition of a molecule. This distinction will be explained in Solved Problem 3.x at the end of this section. [Au: I think we need to explain the distinction here – where the confusion might first arise. Also, we are using the terms molecular and ionic compounds below but students won’t know what we mean by those terms until we get to the Solved Problem at the end of the section. Since we already know what ions are, I think we can say, at this point, something like: “Ionic compounds are formed from a fixed ratio of two or more ions of different elements while molecular compounds are formed from a fixed ratio of two or more atoms of different elements.”. Sound OK?]
1.5 Molecular Compounds
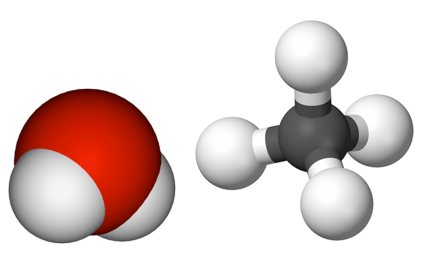
A molecular compoundbinary compounds. Water and methane are both binary molecular compounds. is composed of molecules that contain two or more kinds of atoms [Au: As mentioned, I think we’re just compounding (haha) the confusion if we continue talking about molecules and compounds without distinguishing them. ]. Simple examples include water and methane. A water molecule consists of one oxygen atom and two hydrogen atoms, whereas methane is composed of one carbon atom and four hydrogen atoms (Figure 3.9). Compounds that contain two (and only two) different elements are termed
Solved Problem 3-4: Compare the molecules shown here with those in Figure 3.8. Name the elements and state the number of each kind of element in each of the following molecular compounds: [Unnumbered figure here should show ball-and-stick models (like those in the figure above) of CO, CO2, H2O2, and CH2O.]
Solution: Carbon monoxide consists of one carbon atom and one oxygen atom. Carbon dioxide consists of one carbon atom and two oxygen atoms. Hydrogen peroxide consists of two atoms of hydrogen and two atoms of oxygen. Formaldehyde consists of one atom of carbon, two atoms of hydrogen, and one oxygen atom.
1.6 Ionic Compounds
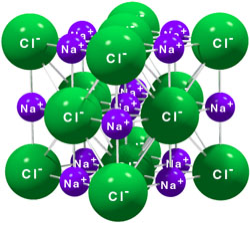
Compounds composed of cations and anions are called ionic compounds. Simple examples include sodium chloride (common table salt) and calcium carbonate (a component of bathtub ring). Ionic compounds contain equal numbers of cations and anions and are thus electrically neutral. A crystal of sodium chloride consists of Na+ cations and Cl- anions in an alternating array called a lattice (Figure 3.10).
Solved Problem 3.5: Compare the definitions of “molecule” and “compound” in this text. Explain how they are similar and how they are different.
“A molecule is a combination of two or more atoms held together in a particular shape or structure by attractive forces.”
“A compound is a substance made up of atoms of two or more elements, combined in a fixed ratio.”
Solution: These definitions are similar in the sense that they describe an assembly of two or more kinds of atoms (or elements). They are different in the sense that the definition of molecule specifies a shape (or arrangement of the atoms) that involves “attractive forces”. To further distinguish these definitions, compare methane (Figure 3.8) and sodium chloride (Figure 3.9).
Methane (CH4) is a molecule consisting of one carbon atom and four hydrogen atoms, linked as shown by forces that we will call “bonds” (described in Section 3.5). In a vessel containing many methane molecules, it is easy to distinguish one from another, because each carbon has only four hydrogen atoms linked to it by these forces. On the other, sodium chloride consists of a lattice of alternating sodium and chloride ions. It is not possible to peer into this lattice and distinguish a “molecule” of sodium chloride, since any given sodium ion has six chloride ions arranged around it (and each chloride has six sodium ions surrounding it). Methane is a molecule that also qualifies as a compound. Sodium chloride is a compound, but it should not be called a “molecule”.
1.7 What are Chemical Bonds?
Molecules and compounds form because atoms are attracted to each other. These attractions are referred to as bonding interactions, or simply chemical bonds. All bonding interactions are the result of attractions between the positively charged nucleus of one atom and negatively charged electrons on another atom. Bonding theory is discussed in more detail in Chapters X and Y, but for now it will be enough to describe two types of bonding interactions: covalent and ionic.
1.8 Covalent Bonds
A covalent bond involves the sharing of electrons between two atoms, most commonly nonmetal atoms. The shared electrons are attracted to the nuclei of both atoms, and the potential energies of these electrons are lowered by the electrostatic interactions with the nuclei. A very simple picture of a covalent bond is shown in Figure 3.11a. When electrons are shared between the two nuclei, attractive electron-nucleus interactions exceed the repulsive nucleus-nucleus and electron-electron forces. The shared electrons hold the two bonding atoms together, resulting in a stable covalent bond. In Chapter 8, we will see that the electrons are most likely to be found between the two nuclei, and we imagine that the electrons are shared between the two atoms.
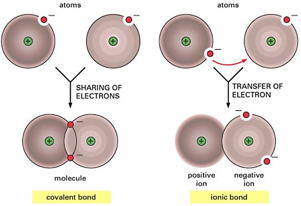
1.9 Ionic Bonds
Whereas covalent bonds result from sharing of electrons between two atoms, the electrostatic attraction between a positively charged cation and a negatively charged anion is termed an ionic bondionic bonding (Figure 3.11b). In a crystal like that in Figure 3.9, electrical forces (termed ionic interactions) hold the cations and anions in place. Each Na+ ion is attracted to all the nearby Cl- ions according to Coulomb’s law (Equation 2.4). Similarly, every Cl- ion is attracted to all the nearby Na+ ions. This attractive force between oppositely charged ions is termed . Note that ionic “bonds” are not directional. This non-directional character favors the formation of ionic lattices with alternating cations and anions (for example, a lattice of Na+ and Cl- ions as in Figure 3.10). By contrast, molecular compounds contain highly directional covalent bonds, and molecular compounds consist of discrete molecules that are not covalently bonded to each other. [Au: I don’t think we should refer to “directional” and “non-directional” without giving some sense of what we mean. Let’s provide a working definition or avoid using these terms until we are ready to explain them in the bonding chapters.]
1.10 Polyatomic Bonds
Many ionic compounds contain an ion that is a group or cluster of two or more covalently bonded atoms with a net charge. These clusters are called polyatomic ions. A simple example is the cyanide ion, CN-, in sodium cyanide (NaCN). The cyanide ion consists of a carbon and nitrogen covalently linked, with an overall negative charge. Other common examples include the bicarbonate ion (HCO3-) in sodium bicarbonate (NaHCO3), also known as baking soda, and ammonium nitrate (NH4NO3), used in agriculture as a high nitrogen fertilizer. In this latter compound, both the ammonium cation (NH4+) and the nitrate anion (NO3-) are polyatomic ions.
1.11 How Do We Represent and Describe Molecules and Compounds?
The simplest way to describe any molecule or compound is with its chemical formula. In such a formula, the atoms of each element are represented by their chemical symbols (a letter or pair of letters) and the number of each type of atom is indicated with a right subscript. The formula for the water molecule, composed of two hydrogen atoms and one oxygen atom, is H2O. Potassium chloride, which consists of one potassium cation and one chloride anion has the formula KCl. The chemical formula for ordinary table sugar (sucrose) is C12H22O11. Some compounds involve a group of atoms that occurs two or more times in the formula. For example, barium hydroxide is a combination of one barium atom and two hydroxide (-OH) groups, and its formula is written Ba(OH)2. Iron acetate consists of one iron atom and three acetate (C2H3O2) groups, and its formula is written Fe(C2H3O2).
Chemical formulas are essential to the description of chemical reactions and also to the calculation of molar masses of compounds. When a new molecule or compound is prepared or discovered, it is critical to establish its chemical formula beyond reasonable doubt.
1.12 Types of Chemical Formulae
Chemists use three different types of chemical formulas – empirical formulas, molecular formulas, and structural formulas – each for different purposes. The relative numbers of atoms in any molecule or compound are expressed in an empirical formula. Thus the empirical formula for acetic acid (the sour taste in vinegar) is CH2O, even though the acetic acid molecule contains two carbons, four hydrogens, and two oxygens. The actual numbers of atoms in a molecule of a compound are represented in the molecular formula. The molecular formula for acetic acid is thus C2H4O2. Similarly the molecular formula for glucose, the simple sugar that provides metabolic energy to the brain, is C6H12O6, whereas the empirical formula is C3H6O3. Note that the molecular formula is usually a whole number multiple of the empirical formula. On the other hand, for some molecules, the empirical formula and the molecular formula are the same. For example, C12H22O11 is both the empirical formula and the molecular formula for sucrose. Why would an empirical formula be used, if for many molecules and compounds it is merely a numerical reduction of the actual formula? We will see in Section 3.11.5 that experimental data on the composition of molecules and compounds is best interpreted in terms of an empirical formula for the material.
Solved Problem 3-6: Give the empirical formula for each of the compounds shown below as molecular formulae: H2O2, C4H8O4, C3H8, N2O5, C6H12O6
Solution: For H2O2, both subscripts can be divided by two, so the empirical formula is HO. For C4H8O4, all four subscripts can be divided by four, so the empirical formula is CH2O. For C3H8, there is no way to divide all the subscripts by the same number, so the empirical formula is C3H8. For N2O5, it is not possible to divide all the subscripts by the same number, so the empirical formula is N2O5. For C6H12O6, all subscripts can be divided by six, so the empirical formula is CH2O
The actual arrangement of bonds in a molecule can be represented with a structural formula. For example, the water molecule consists of an oxygen atom with bonds to two hydrogen atoms and can be represented as
H ⎯ O ⎯ H
The geometric arrangement of bonds in a molecule can also be described in a structural formula. Thus water can be shown as
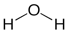
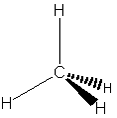
When a molecule is represented this way, it is possible to gain a sense of the shape of the molecule and even its chemical properties. A variant of the structural formula, a perspective drawing, can provide some sense of a molecule’s three-dimensional shape
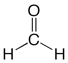
Finally, structural formulas can also portray and distinguish the types of bonds in a molecule. Formaldehyde, for example, contains a carbon with two single bonds to hydrogen atoms and one double bond to an oxygen atom.
A single bond involves a pair of electrons shared between two atoms, whereas a double bond involves two pairs of shared electrons. Double bonds are usually shorter and stronger than single bonds. Triple bonds, such as that in hydrogen cyanide,
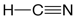
are typically even shorter and stronger than double bonds.
When drawing a structural or perspective formula, how do you know which atom to place in the center of the molecule, and how do you know the angles of bonds around a central atom? The answers to such questions will become apparent in Chapters 8 and 9, when chemical bonds and bonding in molecules and compounds are discussed in much more detail.
How should you choose between empirical, molecular, and structural formulas to represent a molecule? The choice should be based on what information you want to convey, and also how much you know about the compound. If you only know the composition of a compound but not its structure, an empirical formula is your only choice. If the detailed structure of a molecule is known, a molecular or structural formula will convey the most information.
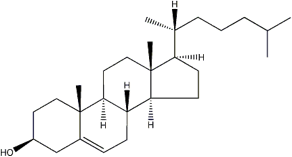
Many of the carbon-based molecules in living things, for example fatty acids, cholesterol, nucleic acids (DNA and RNA), are complicated structures. Seeking simple ways to write these complicated molecules, chemists often use line drawings, a simplification of structural formulas (Figure 3.13). In line drawings, carbon atoms are not labeled, and C⎯H bonds are implied but not drawn. The result is a streamlined, simpler drawing that still conveys the appropriate structural information. All atoms other than carbon and hydrogen are labeled with their element symbols, and hydrogen atoms attached to atoms other than carbon are shown. Single, double, and triple bonds are represented with one, two, and three lines, respectively.