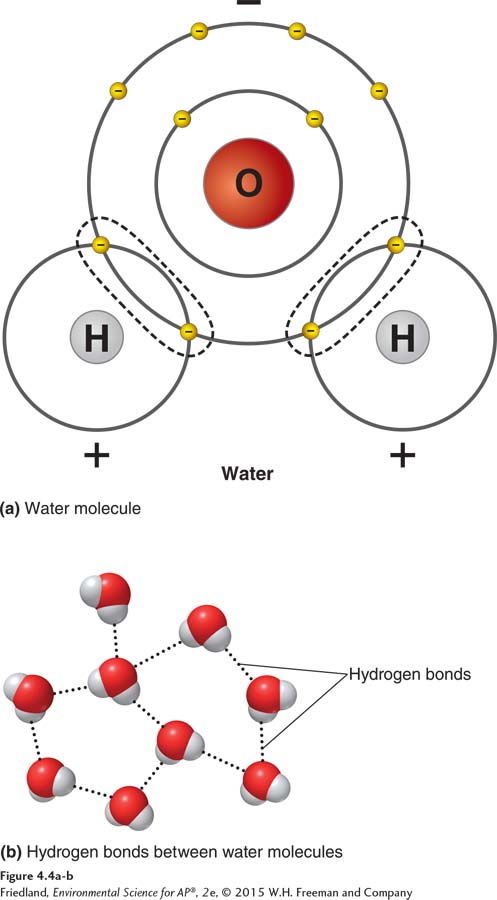
FIGURE 4.4 The polarity of the water molecule. (a) Water (H2O) consists of two hydrogen atoms covalently bonded to one oxygen atom. Water is a polar molecule because its shared electrons spend more time near the oxygen atom than near the hydrogen atoms. The hydrogen atoms thus have a slightly positive charge, and the oxygen atom has a slightly negative charge. (b) The slightly positive hydrogen atoms are attracted to the slightly negative oxygen atom of another water molecule. The result is a hydrogen bond between the two molecules.