4.1 Molecular Structure of Proteins
If you think of a protein as analogous to a word in the English language, then the amino acids are like letters. The comparison is not altogether fanciful, as there are about as many amino acids in proteins as letters in the alphabet, and the order of both amino acids and letters is important. For example, the word PROTEIN has the same letters as POINTER, but the two words have completely different meanings. Similarly, the exact order of amino acids in a protein makes a big di erence because that order determines the protein’s shape and function
4.1.1 Amino acids differ in their side chains.
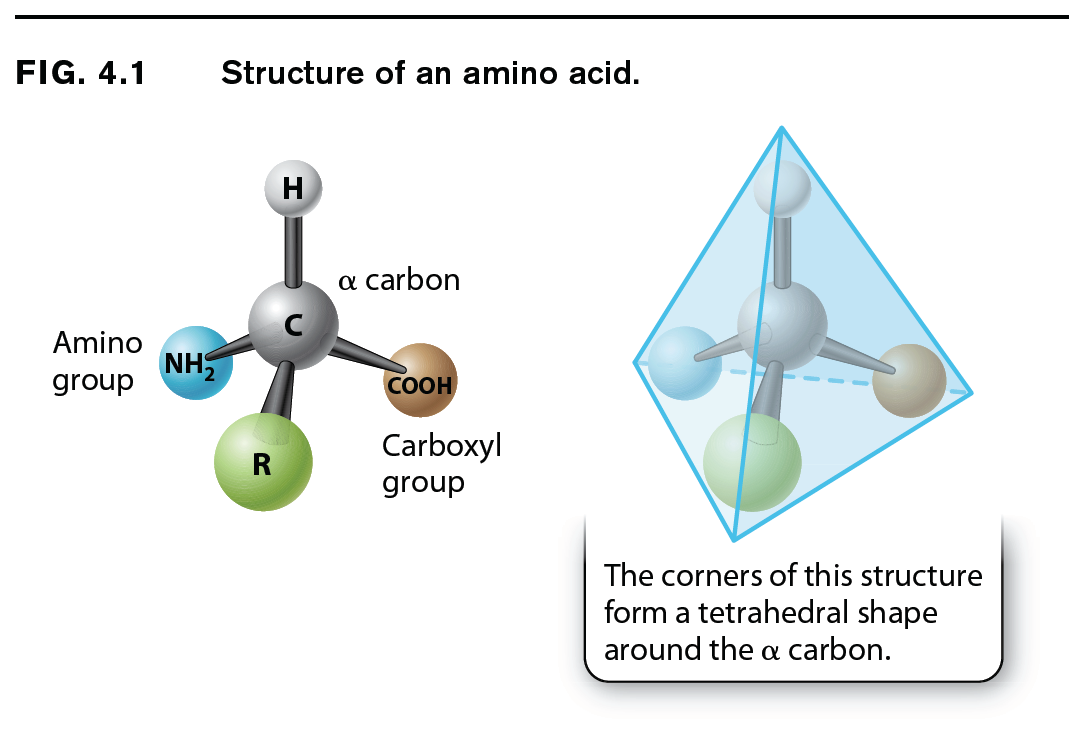
The general structure of an amino acid was discussed in Chapter 2 and is shown again in Fig. 4.1. It consists of a central carbon atom, called the alpha (α) carbon, or connected by covalent bonds to four different chemical groups: an amino group (–NH2 shown in yellow), a carboxyl group (–COOH shown in purple), a hydrogen atom (–H shown in gray), and a side chain or R group (shown in green). The four covalent bonds from the alpha carbon are at equal angles. As a result, an amino acid forms a tetrahedron, a sort of pyramid with four triangular faces. The R groups of the amino acids are also known as side chains, and they differ from one amino acid to the next. They are what make the “letters” of the amino acid “alphabet” distinct from one another. Just as letters differ in their shapes and sounds—vowels like E, I, and O and hard consonants like B, P, and T—amino acids differ in their chemical and physical properties.
The chemical structures of the 20 amino acids commonly found in proteins are shown in Fig. 4.2. The side chains (shown in green) are chemically diverse and are grouped according to their properties, with a particular emphasis on whether they are hydrophobic or hydrophilic, or have special characteristics that might a affect a protein’s structure. Within these broad categories are additional groupings based on whether they are nonpolar or polar, basic or acidic. These properties strongly influence how a polypeptide folds, and hence the three-dimensional shape of the protein.
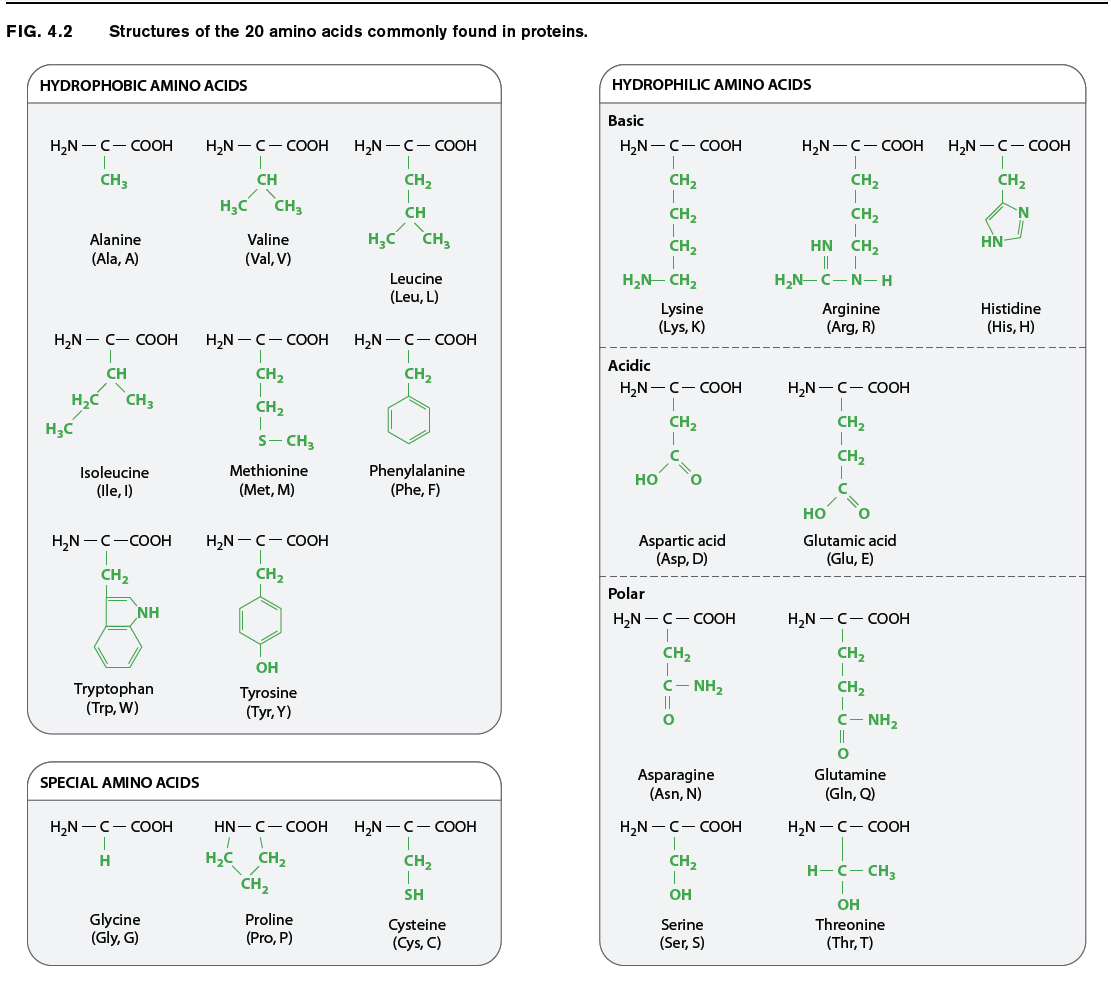
For example, as we saw in Chapter 2, hydrophobic molecules do not readily interact with water and do not easily form hydrogen bonds. Water molecules in the cell therefore tend to form hydrogen bonds with each other instead of with hydrophobic side chains. The aggregation of hydrophobic side chains is also stabilized by weak van der Waals forces (Chapter 2), in which asymmetries in electron distribution create temporary dipoles in the interacting molecules, which are then attracted to each other. The tendency for hydrophilic water molecules to interact with each other and for hydrophobic molecules to interact with each other is the very same tendency that forms oil droplets in water. This is also the reason why most hydrophobic amino acids tend to be buried in the interior of folded proteins, where they are kept away from water.
Amino acids with polar side chains have a permanent charge separation, in which one end of the side chain is slightly more negatively charged than the other. As we saw in Chapter 2, polar molecules are hydrophilic, and they tend to form hydrogen bonds with each other or with water molecules.
The side groups of the basic and acidic amino acids are strongly polar and are also hydrophilic. The side groups of basic amino acids tend to be positively charged at intracellular pH, and those of the acidic amino acids tend to be negatively charged. For this reason, basic and acidic side chains are usually located on the outside surface of the folded molecule. The charged groups can also form ionic bonds (negative with positive) with each other and with other charged molecules in the environment. This ability to bind another molecule of opposite charge is one important way that proteins can associate with each other or with other macromolecules such as DNA.
The properties of several amino acids are noteworthy because of their effect on protein structure. These amino acids include glycine, proline, and cysteine. Glycine is different from the other amino acids because its R group is hydrogen, exactly like the hydrogen on the other side, and therefore it is not asymmetric. All of the other amino acids have four different groups attached to the alpha carbon and are asymmetric. In addition, glycine is nonpolar and small enough to tuck into spaces where other R groups would not fi t. The small size of glycine’s R group also allows for freer rotation around the C–N bond since its R group will not get in the way of the R groups of neighboring amino acids. Thus, glycine increases the flexibility of the polypeptide backbone, which can be important in the folding of the protein.
Proline is also distinctive, but for a different reason. Note how its R group is linked back to the amino group. This linkage creates a kink or bend in the polypeptide chain and restricts rotation of the C–N bond, thereby imposing constraints on protein folding in its vicinity, an e ect the very opposite of glycine’s.
Cysteine makes a special contribution to protein folding through its –SH group. When two cysteine side chains in the same or different polypeptides come into proximity, they can react to form an S–S disulfide bond, which covalently joins the side chains. Such disulfide bonds form cross-bridges that can connect different parts of the same protein or even different proteins. This property contributes to the overall structure of single proteins or combinations of proteins.
4.1.2 Successive amino acids in proteins are connected by peptide bonds.
Amino acids are linked together to form proteins. Fig. 4.3 shows how amino acids in a protein are bonded together. The bond formed between the two amino acids is a peptide bond, shown in red in Fig. 4.3. In forming the peptide bond, the carboxyl group of one amino acid reacts with the amino group of the next amino acid in line, and a molecule of water is released. Note that in the resulting molecule, the R groups of each amino acid point in different directions.
Question 4.2
How many water molecules would be produced in making a polypeptide that is fourteen amino acids long? v69P3wosqLvBa/4W
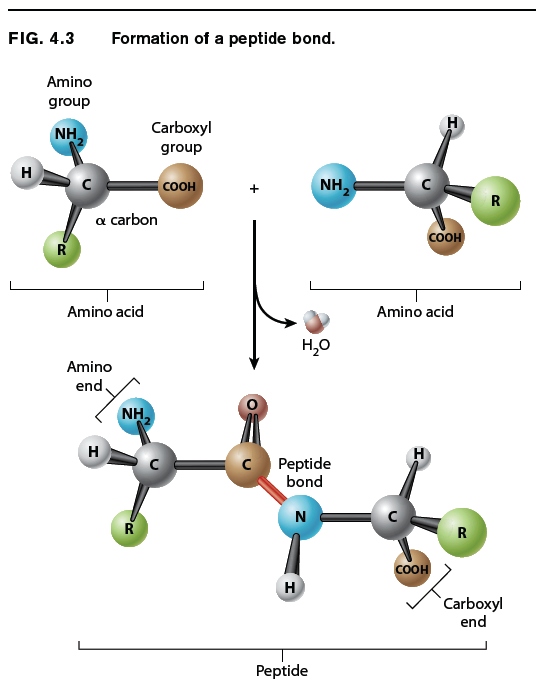
The C=O group in the peptide bond is known as a carbonyl group, and the N–H group is an amide group. Note in Fig. 4.3 that these two groups are on either side of the peptide bond. This arrangement results in the delocalization of electrons, with the e ect that the peptide bond has some of the characteristics of a double bond. The peptide bond is shorter than a single bond, for example, and it is not free to rotate like a single bond. The other bonds are free to rotate around their central axes.
Polymers of amino acids ranging from as few as two to many hundreds share a chemical feature common to individual amino acids: namely, that the ends are chemically distinct from each other. One end, shown at the left in Fig. 4.3, has a free amino group; this is the amino end of the peptide. The other end has a free carboxyl group, which constitutes the carboxyl end of the molecule. More generally, a polymer of amino acids connected by peptide bonds is known as a polypeptide. Typical polypeptides produced in cells consist of a few hundred amino acids. In human cells, the shortest polypeptides are about 100 amino acids in length; the longest is the muscle protein titin, with 34,350 amino acids. The term protein is often used as a synonym for polypeptide, especially when the polypeptide chain has folded into a stable, three-dimensional conformation. Amino acids that are incorporated into a protein are often referred to as amino acid residues.
4.1.3 The sequence of amino acids dictates protein folding, which determines function.
Up to this point, we have considered the sequence of amino acids that make up a protein. This is the first of several levels of protein structure, illustrated in Fig. 4.4. The sequence of amino acids in a protein is its primary structure. The sequence of amino acids ultimately determines how a protein folds. Interactions between stretches of amino acids in a protein form local secondary structures. Longer-range interactions between these secondary structures in turn support the overall three-dimensional shape of the protein, which is its tertiary structure. Finally, some proteins are made up of several individual polypeptides that interact with each other, and the resulting ensemble is the quaternary structure.
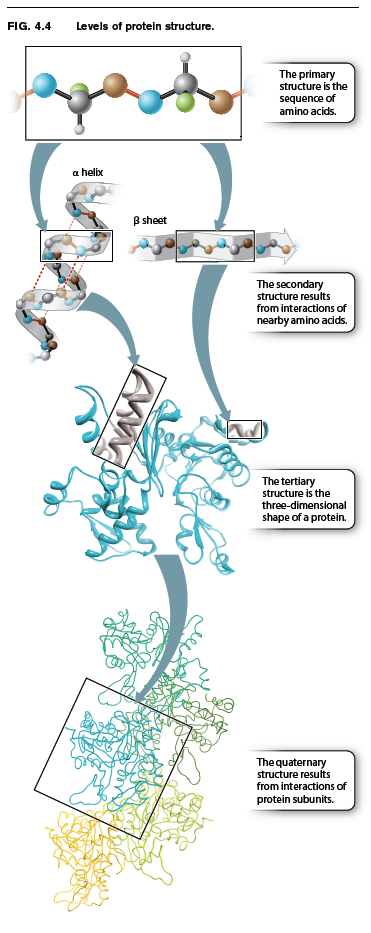
Proteins have a remarkably wide range of functions in the cell, from serving as structural elements to communicating with the external environment to accelerating the rate of chemical reac-tions. No matter what the function of a protein is, the ability to carry out this function depends on the three-dimensional shape of the protein. When fully folded, some proteins contain pockets with positively or negatively charged side chains at just the right positions to trap small molecules; others have surfaces that can bind another protein or a sequence of nucleotides in DNA or RNA; some form rigid rods for structural support; and still others keep their hydrophobic side chains away from water molecules by inserting into the cell membrane.
Question 4.3
1yFLsVwGRmqmBoY//8jen8E2zmi2WKX5gVLZY/BtDtJthTTS3ZIi2usAeY5Au6ScxCtjZPWeFWwh7WO4edDNJkrtcRnUhO5gXAos9RSRIAFZiawOrMpsWhZ0FR2mVEayTpR88y8mcYzPIo89Ipm9aFGGKEHTizxuy7tpKRNRPrvzUZMf7lNSt9Tbwv66m2p98ZVSW0t9NlHlBsobf78mn+4BA964KV+NdmlaXDqFXk/Lnd/NzcA3M5Gp6Jd+2S+7J+R1AmGa6I92/NHhtIck+1iuM6aQ70qMJzFS33znepJjGLQlpvgaFflW19JxkSM4xhHnsaiigDavhMWKacsuJCMfje9xxyGE/GlRZzJWCdiyhd/kaSdPM1/JDlRvcasBzhRiEUvtlVR1kqMVxs+iCyg/FLX/8AmC07ore2Ta8aTWjDfBw3Cytw15WKcPNOxUVQVG4nEabPR3i8c7pbdBQGb2wJ8r/rAXuRxdzVRyC1EUug/3eZCAzSnKOyreU1Wp2rV08k0DtOcD3v8Fh6RmhVJPCLKNz0V8HCvXHe7jWq8KVXq9FaI00ZPX6P37HNpTWls+gCBMuvbVhLumAs3sY4sQKfaFBBNcBM/KRZMqlNn/0Qev5LqU2uKbLA/Rdy/qE3BLaE7iZRkODs2JWR3Tl6+INLOHZeAl0YtzB/vOw4tH+/Da5MPcXCwS31iNP5GVKCW0p7/tA358qu5zpR/QeKWVUnpR0Mbqwd/rYJ7+ayx1ZLQASc02z5MX44Ru/gTjriTPekQ3tGkTmOETsKbv36BB1IYNTPt1Vga0T1sKdO3kYcsKy4UXOv7kUyeKJd+1nbBon7qHaD62vWnughiq/EepB72ZBHO7wrYRah0Lf14kFYduxkv/ZA3XB+pKMUvA8aU0FOYRO1wUvYXNCCZV90eDk1h4udoeStE6bmI/KBNhv9EO38zbO7v4aUnNI4tFdbLdy6X+cTJBcRAnturbAeVF6X+EH6tox19fgVBTMIf6F7C6+DjW5/mjUHe7GW6h+ttEVxzuOAXigoklg4f+pGw5UKmWdxg7m5iysIgF5TEq0CPWEmpwPQ==The sequence of amino acids in a protein (its primary structure) is usually represented by a series of three-letter or one-letter abbreviations for the amino acids (abbreviations for the 20 amino acids are given in Fig. 4.2). By convention, the amino acids in a protein are listed in order from left to right, starting at the amino end and proceeding to the carboxyl end. The amino and the carboxyl ends are di erent, so the order matters. Just as TIPS is not the same word as SPIT, the sequence Thr–Ile–Pro–Ser is not the same peptide as Ser–Pro–Ile–Thr.