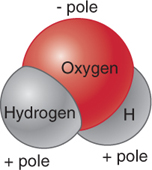
Figure 3.4
A water molecule. A water molecule consists of two hydrogen atoms covalently bonded to one oxygen atom. The oxygen side of the molecule has a weak negative charge, while the hydrogen side has a weak positive charge. These electrical charges create polarity for the water molecule.