1.2 The Weight of Air: Atmospheric Pressure
Explain what causes air pressure and how air pressure changes vertically within the atmosphere.
Gravity keeps molecules in the atmosphere and the oceans pinned to the planet, giving them weight. The gaseous atmosphere and the liquid ocean are both fluids, which means that they flow. But the molecules in the oceans are mostly water and are more closely packed together in a relatively dense liquid form. The molecules in the atmosphere, which are mostly nitrogen and oxygen, are farther apart from one another in their less dense, gaseous state. Nonetheless, the atmosphere has mass. Indeed, the total weight of the atmosphere is estimated to be 5 quadrillion metric tons!
Question 1.2
How high does the atmosphere go?
Ninety-
If you were to travel straight up, how far would you have to travel to get to the top of the atmosphere? It turns out that the answer is not straightforward, because the atmosphere does not end abruptly; instead, it gradually fades. Ninety-
Air Pressure
Molecules in a gaseous state move quickly. They constantly collide with and bounce off one another. At sea level, a single air molecule collides with some 10 billion other molecules each second. It also collides with any other object it comes in contact with, such as trees, the ground, birds, water, and people. Each time a molecule in the air hits any surface, it pushes ever so slightly against it. The collective force (push) of air molecules creates pressure. Air pressure is the force exerted by molecules of air against a surface.
air pressure
The force exerted by molecules of air against a surface.
39
Except for a few locations below sea level (such as the Dead Sea, with an elevation of −427 m [−1,401 ft]), air pressure is greatest at sea level, and it decreases with increasing elevation. To illustrate this point, Figure 1.3 makes the comparison between pressure in a swimming pool and pressure in the atmosphere.
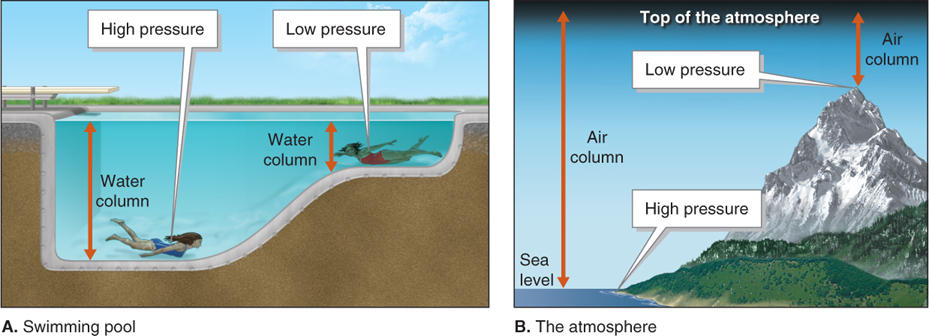
Figure 1.3
 Animation
Animation
Air pressure
http:/
Air Density and Pressure
Because molecules in a gas are far apart, their density can increase as they are compressed. At most times, the density of air is greatest near sea level because the weight of the atmosphere above compresses the air (Figure 1.4).
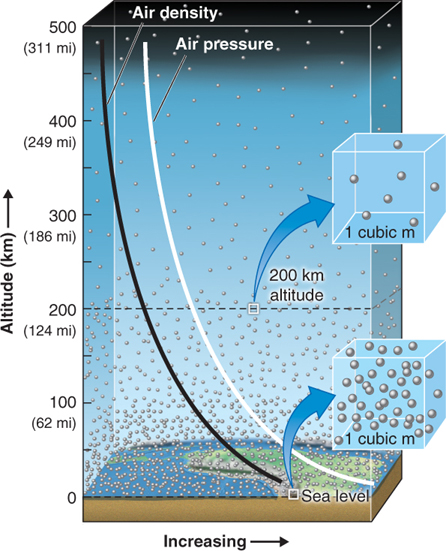
Figure 1.4
Increased air density contributes to air pressure because in denser air, there are more molecules to exert force against objects, creating air pressure. This increased air density is the result of the weight of the overlying mass of air. (Note that, unlike molecules in air, molecules of water in a liquid are already close together and cannot be compressed further.)
Measuring Air Pressure
One way to express air pressure is in kilograms per square centimeter (kg/cm2) or in pounds per square inch (lb/in2 or PSI). At sea level, there is approximately 1 kg pressing on every square centimeter of all objects (or 14.7 lb pressing on every square inch). Put a different way, a column of air that is 1 cm2 in area and extends from sea level all the way up to the top of the atmosphere would, on average, weigh 1 kg (or 14.7 lb for a 1 in2 column of air). (See Section 4.2 for a discussion of air pressure in the context of weather systems.)
40
Because the atmosphere is weighing down on our bodies, you might wonder why we are not crushed by its weight, much like being crushed beneath the weight of a large boulder. The answer is that a large boulder is a solid and would press against us only from above. Air and water, on the other hand, are fluids. They flow around (and inside) our bodies. As a result, the atmosphere (and water, when we are swimming) pushes down, sideways, and up against our bodies. We experience the same pressure from all angles simultaneously. The cells in our bodies exert outward pressure in balance with the inward pressure exerted by the atmosphere, and as a result, we cannot feel the pressure of the atmosphere.
The pressure between our inner ears and the atmosphere is normally in equilibrium. When the outside pressure is changed by driving in mountainous areas or flying, this equilibrium is temporarily disrupted until our ears adjust to the change of pressure. These pressure differences push on the eardrum and can be quite uncomfortable.