3.1 The Hydrologic Cycle and Water
Explain what the hydrologic cycle is and why it is important.
Question 3.1
How do hurricanes weigh trillions of tons yet remain suspended in the air?
Trillions of tons of water can be suspended in the atmosphere because it exists as microscopic droplets of water and ice crystals, which individually are nearly weightless.
Liquid water is heavy: it weighs 1 kg/L (8.3 lb/gal). A single small cumulus cloud, made of microscopic liquid cloud droplets, can contain millions of liters of water and weigh several hundred metric tons. Hurricanes are much larger and weigh trillions of metric tons. How does such weight defy gravity and remain suspended in the atmosphere? The answer to this question lies in the ability of water to shift between the three states, or phases, of matter: solid, liquid, and gas at temperatures found on Earth’s surface.
States of Water: Solid, Liquid, and Gas
Water shifts between solid, liquid, and gaseous states through melting, evaporation, condensation, and freezing. Evaporation is the change in the state of water from liquid to water vapor (a gas), and condensation is the change in state from water vapor to liquid water (Figure 3.2). When liquid water evaporates, it is mixed into the atmosphere as water vapor. Through evaporation, the Sun’s energy lifts trillions of tons of water into the atmosphere, water molecule by water molecule. That water vapor condenses into liquid cloud droplets, which are kept suspended in the atmosphere by updrafts of air. The resulting liquid water then falls back to Earth as precipitation. Precipitation is solid or liquid water that falls from the atmosphere to the ground.
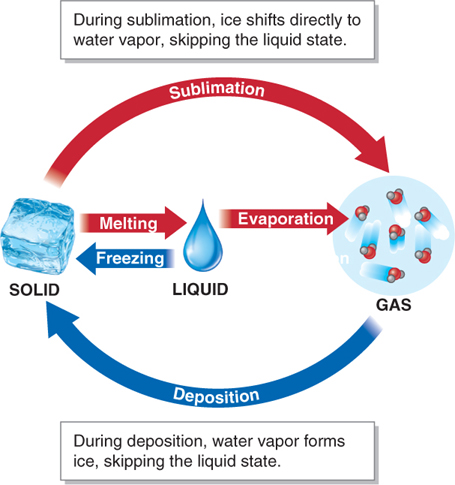
Figure 3.2
evaporation
A change in the state of water from liquid to gas.
condensation
A change in the state of water from gas to liquid.
precipitation
Solid or liquid water that falls from the atmosphere to the ground.
Two additional state changes, which skip the liquid state of water entirely, are possible. During sublimation, ice shifts directly to water vapor. During deposition, water vapor forms ice directly.
96
The Hydrologic Cycle: Water on the Move
The hydrologic cycle is the movement of water within the atmosphere, biosphere, lithosphere, and hydrosphere (Figure 3.3). The hydrologic cycle provides a larger context for the movement of water into and out of the atmosphere.
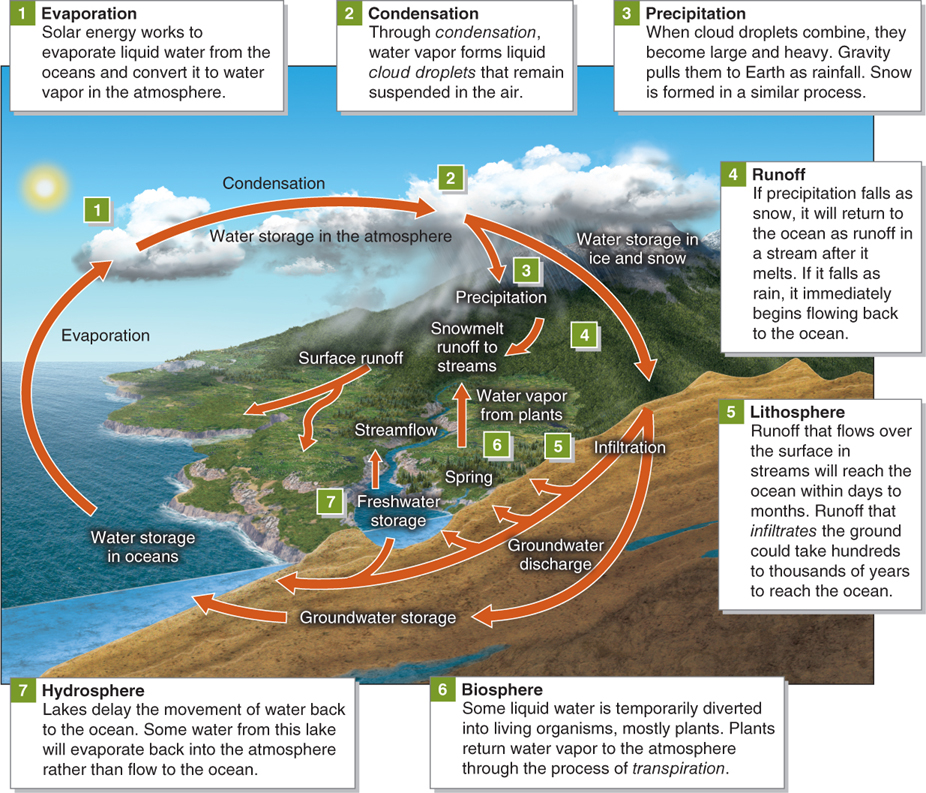
Figure 3.3
 Animation
Animation
Hydrologic cycle
http:/
hydrologic cycle
The movement of water within the atmosphere, biosphere, lithosphere, and hydrosphere.
The hydrologic cycle is entirely solar powered. About 30% of all solar energy absorbed by Earth goes to work in evaporating water from Earth’s surface. Evaporation from the oceans provides about 85% of the water vapor in the atmosphere. Transpiration, the loss of water to the atmosphere by plants, and evaporation from surface streams and lakes account for the remaining 15% of atmospheric water vapor.
transpiration
The loss of water to the atmosphere by plants.
Globally, the amount of water entering the atmosphere is equal to the amount leaving it. Over land, however, precipitation exceeds evaporation. Conversely, across most of the surface of the oceans, evaporation exceeds precipitation. The water removed from the oceans through evaporation is returned to the oceans by stream runoff from the continents or by rainfall directly on the oceans.
Properties of Water
Water readily shifts between its three states or phases at temperatures commonly found in the lower atmosphere. What are the properties of water that allow it to do this?
Water molecules are formed by the covalent bonding of hydrogen and oxygen atoms, as illustrated in Figure 3.4. Individual water molecules attach to one another because of their electrical polarity: The hydrogen end of a water molecule has a weak positive charge, and its oxygen end has a weak negative charge. One water molecule’s positive end attaches to another water molecule’s negative end, forming a hydrogen bond. The covalent bonds holding the atoms together to form a water molecule are far stronger than the hydrogen bonds that join water molecules.
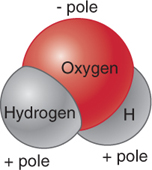
Figure 3.4
hydrogen bond
The bond between water molecules that results from the attraction between one water molecule’s positive end and another’s negative end.
97
The state of water depends on the number of hydrogen bonds in it. Ice has more hydrogen bonds than liquid water or water vapor. The hydrogen bonds in ice create a six-
Water molecules join to other water molecules through cohesion (by means of hydrogen bonds), and water molecules join to other objects through adhesion. Cohesion is only one of the properties of water that are central to its roles in so many physical processes on Earth (Figure 3.5).
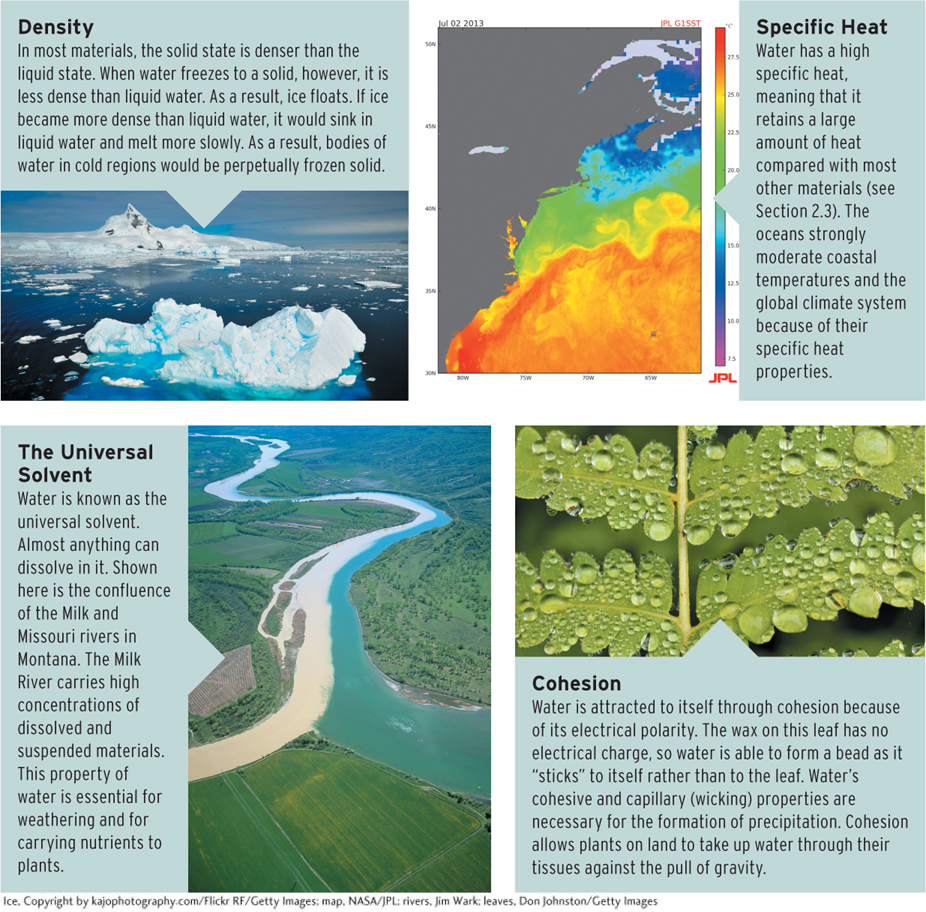
Figure 3.5
Latent Heat of Water: Portable Solar Energy
Water changes its state when energy is either added to or removed from water molecules. We learned in Section 2.2 that 1 calorie of energy raises 1 g of liquid water 1°C. So it makes sense that if we had 1 g of water (1 cm3 or 1 ml), and we added 1 calorie to it, the temperature of the water would rise from 98°C to 99°C. We could add one more calorie, and the temperature would rise to 100°C (212°F, water’s boiling temperature at sea level). The water would begin boiling and vaporizing.
Logically, our next calorie of energy should raise the temperature of our gram of boiling water to 101°C. But that is not what happens. When we add one more calorie, the temperature of the water remains at 100°C. If we continue to add calories, the water temperature still remains at 100°C. These calories absorbed by the water are referred to as latent heat. Latent heat is the energy that is absorbed or released during a change in the state of a substance, such as the evaporation or condensation of water.
latent heat
Energy that is absorbed or released during a change in the state of a substance, such as during evaporation or condensation of water.
98
In all, the state change of liquid water at 100°C to water vapor at 101°C requires 541 calories of energy. Five hundred and forty calories are required to vaporize the water, and 1 calorie is required to raise the water vapor temperature from 100° to 101°C. Those 540 calories work to break the hydrogen bonds in liquid water—
The energy used to melt ice is called the latent heat of melting. Only 0.5 calorie of sensible heat is required to raise the temperature of ice 1°C because ice has a lower specific heat than liquid water. The energy used to vaporize liquid is called the latent heat of vaporization (Figure 3.6). Similarly, as water vapor condenses, it must release its stored latent heat as latent heat of condensation.
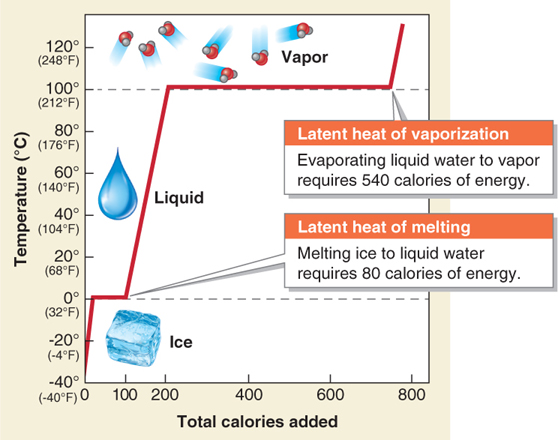
Figure 3.6
Yet water’s temperature does not have to be raised to 100°C for it to evaporate. We can prove this by spraying a warm summer sidewalk with a garden hose. The water will not boil, but it will quickly evaporate. Even after winter rainstorms when it is cold outside, puddles on the streets and sidewalks evaporate.
When a photon of solar energy is absorbed by an individual water molecule at the surface of a body of water, the molecule may obtain enough energy to break the hydrogen bonds with its neighbors and evaporate. The energy involved in this process is called the latent heat of evaporation. This evaporation process occurs in natural outdoor settings with temperatures well under 100°C. Boiling, in contrast, occurs when the water temperature is 100°C, as in a pot of water on the stove or a natural hot spring.
Depending on the temperature of the water, the amount of energy required to evaporate it varies from about 540 calories to 600 calories per gram of water. In other words, it takes more calories to evaporate water at temperatures below 100°C (Figure 3.7).
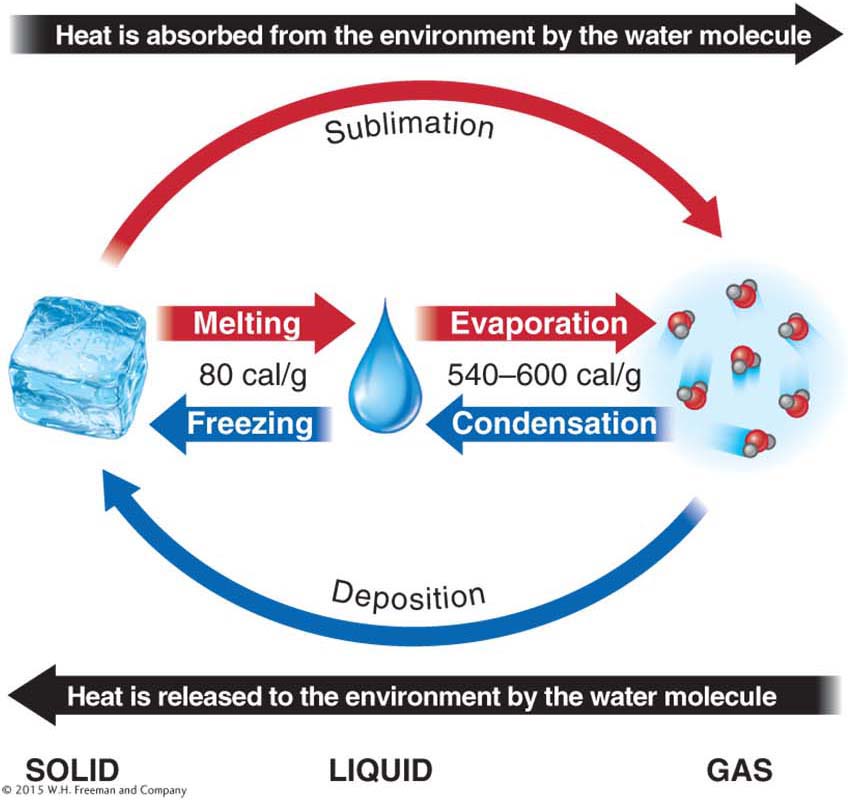
Figure 3.7
Evaporation is a cooling process. As we have just seen, up to 600 calories per gram of water are absorbed from the environment during the process of evaporation. Thus the temperature of the surrounding environment drops as heat is absorbed by water molecules. Your body feels cooler when your sweat evaporates on a hot summer day because it takes up to 600 calories of heat to evaporate 1 g of sweat. Many, but not all, of those calories come from your body. (Note that the calories we are using for these measurements are not the same as the calories used to measure the energy content of food, which are given in kilocalories or kcal. One kilocalorie is equal to 1,000 calories.)
99
Condensation is a warming process. About 600 calories of latent heat per gram of water are released to the surrounding environment as water vapor becomes liquid water.
Evaporation and condensation are always occurring at the same time in Earth’s lower atmosphere and surface waters. When evaporation exceeds condensation, net evaporation is occurring, and the air is cloud free. When condensation exceeds evaporation, net condensation is occurring, and clouds form. Net condensation occurs when air has reached saturation: that is, the point at which the air’s water vapor content is equal to the air’s water vapor capacity.
saturation
The point at which an air parcel’s water vapor content is equal to its water vapor capacity.