3.2 Atmospheric Humidity
Distinguish between the different ways of expressing atmospheric humidity and explain how each works.
Humidity is the amount of water vapor in the atmosphere. Only water vapor contributes to humidity. Condensation and precipitation lower humidity because they remove water vapor from the atmosphere.
humidity
The amount of water vapor in the atmosphere.
As we have seen, evaporation from the oceans accounts for most of the water vapor in the atmosphere. The most humid air is found in the tropics near warm oceans, where evaporation rates are high (Figure 3.8). In tropical rainforests, however, most of the humidity comes from transpiration by plants, not evaporation. Together, evaporation and transpiration create a single process of moving water to the atmosphere, called evapotranspiration.
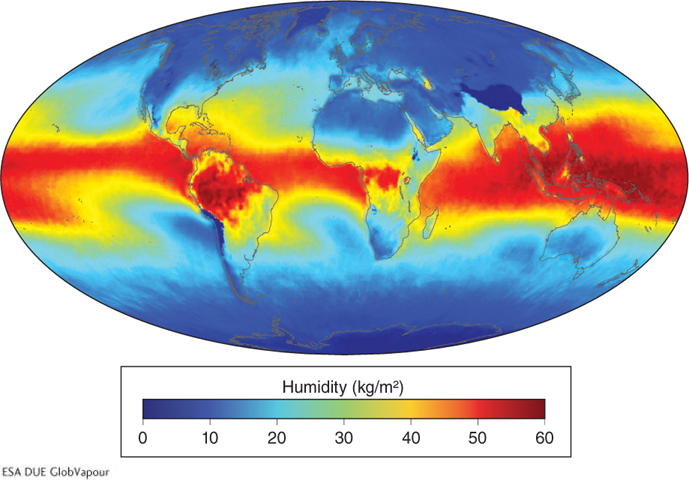
Figure 3.8
evapotranspiration
The combined processes of evaporation and transpiration.
Hygrometers of several types are used to measure humidity. Sling psychrometers, for example, are large, hand-
hygrometer
An instrument used to measure humidity.
The Heat-Index Temperature
As the saying goes, “It’s not the heat, it’s the humidity.” Heat waves are usually associated only with high temperatures, but high humidity also plays an important role in the effects of heat waves on people. As we saw in Section 2.2, the heat-
Heat waves are the number one meteorological killer in the United States: Each year, about 400 people lose their lives because of heat waves. When a heat wave gripped the Chicago and Milwaukee areas in 1995, over 750 people died. In August 2003, as many as 70,000 people died in Europe as a result of exceptionally high heat-
100
The Many Names for Humidity
Many different units of measure are used to express humidity. All measures of humidity gauge how much water vapor is in the air. Here we will examine four of these measures: vapor pressure, specific humidity, relative humidity, and the dew point.
Vapor Pressure
Vapor pressure expresses the water vapor content of air by the amount of pressure it creates. Vapor pressure is the portion of air pressure exerted exclusively by molecules of water vapor.
vapor pressure
The portion of air pressure exerted exclusively by molecules of water vapor.
In Section 1.2, we learned that the weight of the atmosphere exerts 1 kg of pressure on every square centimeter (1 kg/cm2 or 14.7 lb/in2) of all objects at sea level. A millibar (mb) is a measure of atmospheric pressure that is conceptually identical to kilograms per square centimeter. Both units refer to the force exerted by air molecules as they push against a surface. Millibars are commonly used by meteorologists and other scientists because they are finer units that can express small changes in atmospheric pressure. (A hectopascal or hPa is the metric equivalent of a millibar.)
millibar (mb)
A measure of atmospheric pressure; average sea level pressure is 1013.25 mb.
The average weight of the atmosphere at sea level exerts 1013.25 mb of pressure, and a portion of this pressure is caused by molecules of water vapor. Imagine stuffing a water bottle into a backpack already filled with books. The extra “pressure” exerted by the water bottle in addition to the pressure exerted by the books and other items in the backpack would be equivalent to vapor pressure.
Evaporation and condensation occur continuously throughout the atmosphere, but net condensation occurs only when air is saturated. The vapor pressure at which saturation occurs, called the saturation vapor pressure, varies with air temperature. As shown in Figure 3.9, for every 10°C increase in air temperature, saturation vapor pressure approximately doubles. In other words, as air temperature increases, more water vapor must be added for saturation to occur.
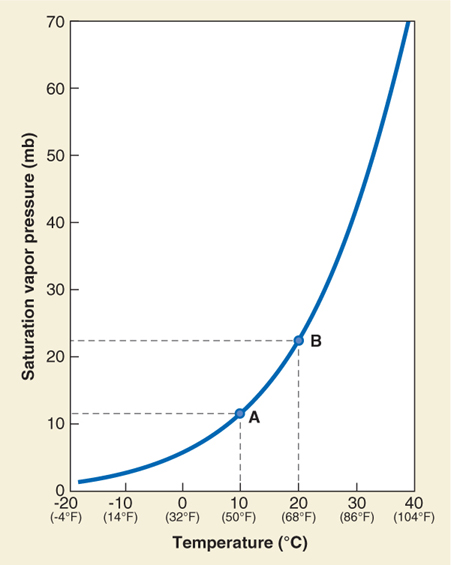
Figure 3.9
saturation vapor pressure
The vapor pressure at which saturation occurs.
Specific Humidity
Another measure of humidity evaluates the water vapor content of an air sample by mass. Specific humidity is the water vapor content of the atmosphere, expressed in grams of water per kilogram of air (g/kg):
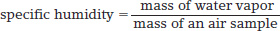
specific humidity
The water vapor content of the atmosphere, expressed in grams of water per kilogram of air (g/kg).
If, for example, there are 10 g of water vapor in a kilogram of air, then the specific humidity is 10 g/kg. Specific humidity changes only when water vapor is added to or removed from the atmosphere. Condensation lowers specific humidity, and evaporation raises it.
Relative Humidity
Relative humidity is a common way of expressing humidity that you may already be familiar with. Relative humidity (RH) is defined as the ratio of water vapor content to water vapor capacity, expressed as a percentage.
relative humidity (RH)
The ratio of water vapor content to water vapor capacity, expressed as a percentage.
Temperature and relative humidity are inversely related (they change in opposite directions). Warm air has a greater water vapor capacity than cold air. Therefore, if the air temperature rises, the water vapor capacity of the air also rises. In terms of its water vapor capacity, the air will have proportionally less water vapor as the temperature increases (as long as no water vapor is evaporated into the air so the level of water vapor remains constant). It may sound right to say that warm air can “hold” more water vapor than cold air—
If the temperature of air is lowered, the water vapor capacity of the air decreases. As cooling continues, the air will eventually become saturated. When air is saturated, the relative humidity is 100%. When the relative humidity is 100%, clouds and precipitation may form. Figure 3.10 summarizes the general relationship between temperature and relative humidity graphically.
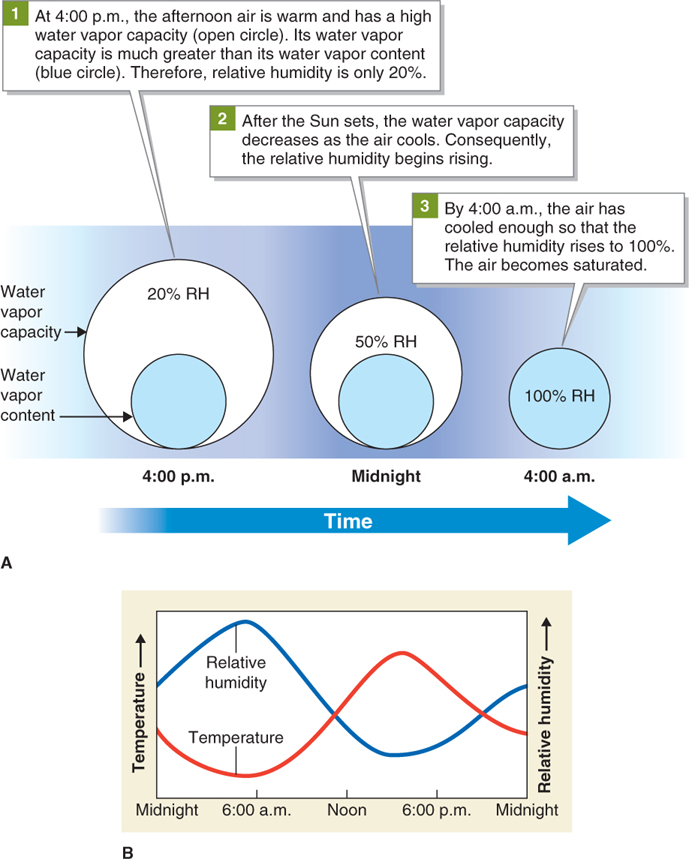
Figure 3.10
101
The following examples use water vapor content (expressed as vapor pressure) to illustrate quantitatively how relative humidity changes. First, we can express relative humidity using this formula:
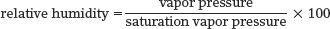
We will apply this relative humidity formula to an air parcel, defined as a body of air with uniform humidity and temperature.
air parcel
A body of air of uniform humidity and temperature.
Imagine a parcel of air at 30°C with a vapor pressure of 22 mb. At this temperature, the saturation vapor pressure is 42 mb (refer back to Figure 3.9). With these numbers, we can calculate the relative humidity (RH) of the air parcel:
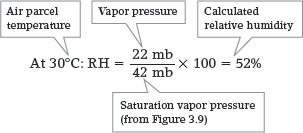
If we lower the temperature of the air parcel to 25°C, the relative humidity will increase:

Notice that the 22 mb vapor pressure in the air parcel did not change because we did not add or remove water vapor from the atmosphere. Only the air temperature changed. Crunch the Numbers further explores how temperature changes the relative humidity of air.
CRUNCH THE NUMBERS: Calculating Humidity
CRUNCH THE NUMBERS: Calculating Humidity
Read the description of relative humidity above and use it as a guide as you work through these problems. Assume that vapor pressure does not change.
Calculate the relative humidity for a 20°C air parcel with a vapor pressure of 10 mb. (Hint: First use Figure 3.9 to find the saturation vapor pressure for 20°C air.)
Calculate the new relative humidity for that same parcel of air when the temperature rises to 30°C.
If the air temperature were lowered to 9°C, what would the new relative humidity be?
102
Polar air often has high relative humidity because it is cold. Warm tropical air also has high relative humidity because immense amounts of water are evaporated from warm tropical oceans. The high vapor pressure of tropical air creates high relative humidity even when the temperature is high, as shown in Figure 3.11.
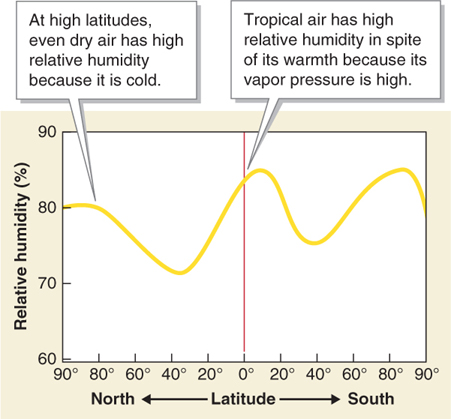
Figure 3.11
Changes in vapor pressure also affect relative humidity. Increases in evaporation resulting in increases in relative humidity happen in many different atmospheric situations outside of the tropics as well. The two examples in Picture This show how air can become saturated by raising the vapor pressure.
Picture This

Saturation by Raising Vapor Pressure
Relative humidity can be increased to 100% by a rise in the vapor pressure so that it equals the saturation vapor pressure. This happens more readily when the air is cold and has a low saturation vapor pressure. Remember, water vapor is invisible. The “steam” (condensation) seen in the photos above is a cloud composed of liquid cloud droplets.
Consider This
Question 3.2
2vQJF3Yx6dS3nkdnPhwG9vFKbnp3RQsz4u70D+fhMoraQIMmiA9gYe4UZEXstKgmuujo6h9U0X05/Tb1kRN7OFkmAM+rwNXzocwTbksv5juJesj9wPJv4IROxyH2/j/5x4uPD/eHIc4=Question 3.3
/oNwv4qdsQU8WbFrBq8uFSDBTj+lCkTQKfLeE8bXP0ubgKK9Hs4JmZYX57BJBvm2L+vrF213ipQWJBYvIUKQSCkfJvWi4N/lseEkdOF8J1F/JvAjW3phgQ==
Question 3.4
Why does water form on the outside of a glass containing a cold drink?
The cold drink has lowered the air temperature near the container’s surface to the dew point, resulting in condensation.
The Dew Point
Have you ever wondered why the outside of a container of an ice-

Figure 3.12
dew point
(or dew-
103
The air parcel we considered became saturated when its temperature was lowered to 19°C. For that parcel of air, therefore, the dew point was 19°C. The dew point is a reliable indicator of humidity because in unsaturated air, the dew point does not change as air temperature changes.
Dry air has a low dew point, and humid air has a high dew point. Once dew points are above about 20°C (70°F), the humidity becomes uncomfortable for people. Figure 3.13 shows the geographic pattern of humidity in the United States using dew points.
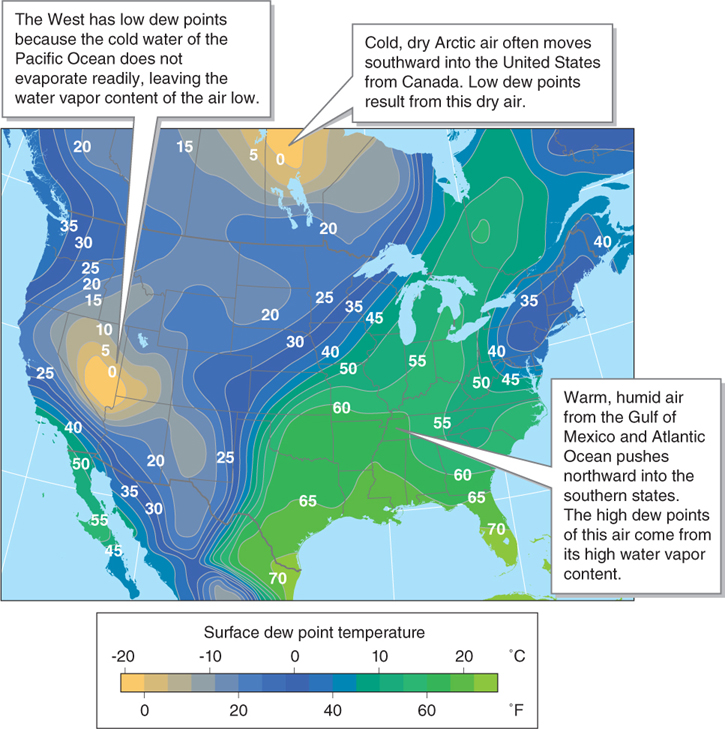
Figure 3.13