17.2 Biological Treatments
A person diagnosed with a mental disorder might be treated by biological means (most often drugs), psychological means (psychotherapy of one form or another), or both. Biological treatments attempt to relieve the disorder by directly altering bodily processes. In the distant past, such treatments included drilling holes in the skull to let out bad spirits and bloodletting to drain diseased humors. Today, in decreasing order of extent of use, the three main types of biological treatments are drugs, electroconvulsive shock therapy (ECT), and psychosurgery.
Drugs
A new era in the treatment of mental disorders began in the early 1950s when two French psychiatrists, Jean Delay and Pierre Deniker (1952), reported that they had reduced or abolished the psychotic symptoms of schizophrenia with a drug called chlorpromazine. Today, a plethora of drugs is available for treating essentially all major varieties of mental disorders.
Drugs for mental disorders have been far from unmixed blessings, however. They are not magic bullets that zero in on and correct a disordered part of the mental machinery while leaving the rest of the machinery untouched. Drugs used to treat mental disorders nearly always produce undesirable side effects. A few of the antianxiety drugs are addictive, and the attempt to withdraw from them sometimes produces symptoms worse than those for which the drug was prescribed. As you read of the three categories of drugs described below, notice their drawbacks as well as their benefits.
Antipsychotic Drugs
Antipsychotic drugs are used to treat schizophrenia and other disorders in which psychotic symptoms (such as hallucinations and delusions) predominate. Chlorpromazine (sold as Thorazine) was the first such drug, but now there are many others. Well-designed experiments have shown repeatedly that such drugs reduce and in some cases abolish the hallucinations, delusions, and bizarre actions that characterize the active phase of schizophrenia and that they reduce the need for hospitalization (Barnes & Marder, 2011; Stroup et al., 2006). All antipsychotic drugs in use today decrease the activity of the neurotransmitter dopamine at certain synapses in the brain, and that effect is believed to be responsible, directly or indirectly, for the reduction in psychotic symptoms.
668
5
What is known about the mechanisms, effectiveness, and limitations of drugs used to treat schizophrenia, generalized anxiety, and depression?
Since the early 1990s, much research and discussion has centered on reputed differences between two classes of antipsychotic drugs—the so-called typical and atypical antipsychotics. The typical drugs—of which haloperidol is most used—were the ones first developed, and the atypical drugs—including olanzapine and risperidone—are newer. Early research on the atypical drugs, done mostly by the pharmaceutical companies themselves, suggested that they were more effective than the older drugs in reducing psychotic symptoms and that they produced fewer harmful side effects. More recent, unbiased, large-scale studies conducted with public funds, however, have questioned these contentions (Carpenter & Buchanan, 2008; Crossely et al., 2010; Stargardt et al., 2008). At present there is no strong evidence that the atypical drugs are better overall than the less-expensive typical drugs. In terms of their biochemical mechanisms, both classes of drugs work by decreasing dopamine activity, although the atypical drugs also affect receptors for other neurotransmitters such as serotonin (Waddington et al., 2011).
All antipsychotic drugs have unpleasant and damaging side effects. They can produce dizziness, confusion, nausea, dry mouth, blurred vision, heart rate irregularities, constipation, weight gain, heightened risk for diabetes, sexual impotence in men, and disrupted menstrual cycles in women (Haddad & Sharma, 2007; Geddes et al., 2011; Stroup et al., 2012). They also interfere with motor-control processes in the brain and sometimes produce symptoms akin to Parkinson’s disease, including shaking and difficulty in controlling voluntary movements. Many patients who take such drugs for many years develop a serious and often irreversible motor disturbance called tardive dyskinesia, manifested as involuntary jerking of the tongue, face, and sometimes other muscles.
Given such effects, it is no wonder that many patients diagnosed with schizophrenia stop taking the drugs as soon as the psychotic symptoms decline, or sometimes even before. Clinicians usually regard such “failure to comply” as a serious problem, as there is indeed evidence that, for many patients, such non-compliance means that they are more likely to have another psychotic episode and require readmission to the hospital. A fact ignored by many clinicians, however, is that a significant number of patients who stop taking the drugs do quite well without them (Harrow & Jobe, 2007). These patients are frequently unknown to the clinicians because, not needing further treatment, they do not return to the clinic. As far we can tell—and we have looked—there have been no research studies designed to test the long-term consequences of living without the drugs compared to living with them, or to determine which patients really need the drugs and which ones can do without them, or to test the logically plausible hypothesis that long-term use of the drugs might alter the brain in ways that prevent eventual full recovery from schizophrenia. The mental health industry, whose research is financed mostly by pharmaceutical companies, has shown little interest in these questions.
Antianxiety Drugs
Drugs used primarily to treat anxiety are commonly referred to as tranquilizers. At one time, barbiturates such as phenobarbital were often prescribed as tranquilizers, and many people became seriously addicted to them. During the 1960s, barbiturates were replaced by a new, safer group of antianxiety drugs belonging to a chemical class called benzodiazepines [běn’-zō-dī-ăz’-ǝ-pēns], including chlordiazepoxide (sold as Librium) and diazepam (sold as Valium). According to some estimates, by 1975 more than 10 percent of adults in the United States and Western Europe were taking these drugs on a regular basis (Lickey & Gordon, 1991; Lipman, 1989).

Biochemically, benzodiazepines appear to produce their tranquilizing effects by augmenting the action of the neurotransmitter GABA (gamma-aminobutyric acid) in the brain (Hefti, 2005). GABA is the brain’s main inhibitory neurotransmitter, so its increased action decreases the excitability of neurons almost everywhere in the brain. Side effects of benzodiazepines at high doses include drowsiness, a decline in motor coordination, and a consequent increase in accidents. More important, the drugs enhance the action of alcohol; an amount of alcohol that would otherwise be safe can produce a coma or death in people taking a benzodiazepine. In addition, benzodiazepines are now known to be at least moderately addictive, and very unpleasant withdrawal symptoms—sleeplessness, shakiness, anxiety, headaches, and nausea—occur in those who stop taking them after having taken high doses for a long time (Bond & Lader, 1996).
669
Early research with benzodiazepines tended to show that they were highly effective in relieving generalized anxiety. More recent, more carefully controlled studies, however, have shown them to be of questionable effectiveness (Martin et al., 2007). Such studies have revealed that more than half of the people who were randomly assigned to the benzodiazepine condition dropped out either because of lack of anxiety relief or because of intolerance of the side effects (Martin et al., 2007). Overall, in the more recent studies, those on a placebo (a pill with no active chemicals) did nearly as well as those on a benzodiazepine.
Today benzodiazepines are still often used to treat generalized anxiety disorder and panic disorder, but are rarely used for other anxiety disorders. Their use for all anxiety disorders has declined partly because of growing recognition of their harmful side effects and partly because of evidence that anxiety may be better treated with antidepressant drugs, in the SSRI class, described below (Mathew & Hoffman, 2009). The effectiveness of antidepressant drugs in treating anxiety disorders is consistent with the evidence, discussed in Chapter 16, for a close biological relationship between anxiety and depression.
Antidepressant Drugs
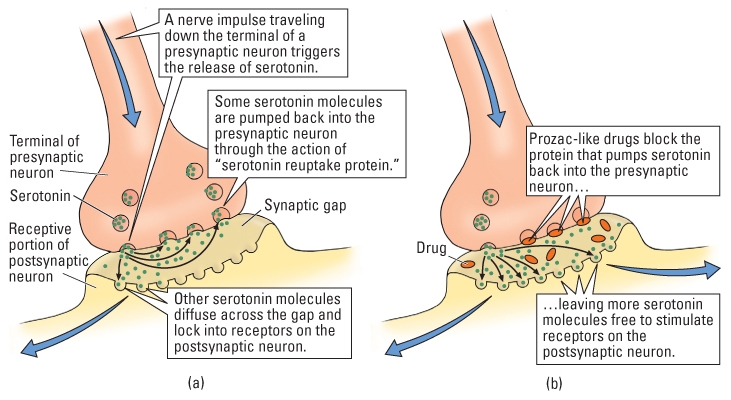
From the 1960s into the mid-1980s, the drugs most commonly used to treat depression belonged to a chemical class referred to as tricyclics, of which imipramine (sold as Tofranil) and amitriptyline (sold as Elavil) are examples. Tricyclics block the normal reuptake of the neurotransmitters serotonin and norepinephrine into presynaptic neurons after their release into the synapse, thereby prolonging the action of the transmitter molecules on postsynaptic neurons (Hefti, 2005). Beginning in the mid-1980s, a newer class of antidepressants, referred to as selective serotonin reuptake inhibitors (SSRIs), which block the reuptake of serotonin but not that of other monoamine transmitters, overtook the tricyclic drugs as the first line of treatment for depression (Gitlin, 2009). Among the most-often prescribed of these drugs are fluoxetine (Prozac), citalo-pram (Celexa), and sertraline (Zoloft). To visualize how such drugs act at the synapse, see Figure 17.1.
Many research studies have demonstrated that the tricyclics and SSRIs are about equally effective in treating depression (Carr & Lucki, 2011). With either type of drug, approximately 50 percent of people suffering from major depression show a clinically significant improvement in mood, compared with about 30 percent who improve over the same time period if given a placebo (inactive substance) instead (Hollon et al., 2002). Antidepressants are even less effective for people with mild or moderate depression (Hegerl et al., 2012). The SSRIs are preferred because of their milder side effects. Tricyclics are much more likely to be fatal if taken in overdose than are SSRIs, and they are also more likely to produce such disruptive and discomforting side effects as fatigue, dry mouth, and blurred vision. Still, in some people, the SSRIs do produce side effects such as reduced sexual drive, headache, nausea, and diarrhea (Gitlin, 2009; Hollon et al., 2002; Uher et al., 2011). Despite the drawbacks, many patients are advised to continue taking antidepressants even after a clinical episode lifts to maintain the drug’s beneficial effects.
670
As noted in Chapter 16, the effects of antidepressant drugs on neurotransmitters occur immediately, but the antidepressant effects take several weeks to develop. This fact suggests that some gradual process—maybe including the growth of new neurons in the brain—underlies the therapeutic effect (Perera et al., 2008). However, despite many theories and much study, researchers still do not know just how antidepressants reduce depression.
Placebo Effects
When drugs are tested for their effectiveness in treating disorders, comparisons are made ideally among at least three different treatment conditions: no treatment, placebo, and drug. A placebo (discussed in Chapter 2) is an inactive substance that is indistinguishable in appearance from the drug. Such experiments are conducted in a double-blind manner (also discussed in Chapter 2)—that is, in such a way that neither the patients nor the researchers who evaluate them are told who is receiving the drug and who is receiving the placebo. Experiments of this sort allow researchers to separate out three different categories of effects through which people improve:
6
What evidence suggests that only a small percentage of the improvement following drug treatment for depression results from the chemical effects of the drug? Why might the power of suggestion be especially effective in the treatment of depression?
- Spontaneous remission effect refers to any improvement shown by those who receive no treatment.
- Placebo effect refers to any improvement shown by those receiving the placebo that goes beyond the improvement shown by those receiving no treatment.
- Drug effect refers to any improvement shown by those receiving the drug that goes beyond the improvement shown by those receiving the placebo.
When experiments of this sort are conducted with antianxiety or antidepressant drugs, the general finding is that most of the improvement results from spontaneous remission and the placebo effect; only a small amount of improvement can be attributed to the drug itself (Kirsch, 2000; Wampold et al., 2005). For example, in an analysis of such experiments for a variety of antidepressant drugs, Irving Kirsch concluded that about 25 percent of the improvement in those receiving the drug could be attributed to spontaneous remission, about 50 percent could be attributed to the placebo effect, and 25 percent could be attributed to the drug’s chemical effects (Kirsch & Sapirstein, 1998).
Why is the placebo effect so powerful in treating depression? As discussed in Chapter 16, depression is characterized by feelings of helplessness and hopelessness. In fact, for some people those feelings are the disorder. Simply participating in a treatment program—meeting regularly with someone who seems to care and who offers the patient what is believed be a useful drug—may restore feelings of control and hope and produce expectations of improvement. These feelings and expectations may promote life changes, such as improved self-care and more involvement with friends that lead to further improvement in mood. All such effects, which derive from the belief that one’s disorder is being treated, contribute to the placebo effect. From this point of view it is really not surprising that a believable placebo may be an effective treatment for depression.
671
Other Biologically Based Treatments
The increased use of drugs, coupled with the increased understanding and acceptance of psychotherapy, has led to the abandonment of most nondrug biological therapies for mental disorders. Two such treatments still used, however, are electroconvulsive therapy and, in very rare cases, psychosurgery. Psychiatrists are also pioneering newer treatments such as deep brain stimulation and transcranial magnetic stimulation.
Electroconvulsive Therapy
7
Under what conditions and how is ECT used to treat depression?
Electroconvulsive therapy, or ECT, is used primarily in cases of severe depression that does not respond to psychotherapy or antidepressant drugs. To the general public this treatment often seems barbaric, and indeed it once was a brutal treatment. The brain seizure induced by the shock would cause muscular contractions so violent that they sometimes broke bones. Today, however, ECT is administered in a way that is painless and quite safe (Keltner & Boschini, 2009; Lisanby, 2007). Before receiving the shock, the patient is put under general anesthesia and given a muscle-blocking drug so that no pain will be felt and no damaging muscle contractions will occur. Then an electric current is passed through the patient’s skull, triggering a seizure in the brain that lasts approximately 1 minute. Usually such treatments are given in a series, one every 2 or 3 days for about 2 weeks.
According to most estimates, somewhere between 50 percent and 80 percent of people who are suffering from major depression and have not been helped by other forms of treatment experience remission with ECT (Hollon et al., 2002; Lisanby, 2007; Loo, 2010). In some cases, the remission is permanent; in others, depression recurs after several months or more, and then another series of treatments may be given.
Nobody knows how ECT produces its antidepressant effect. In nonhuman animals, such shocks cause immediate release of all varieties of neurotransmitters, followed by longer-lasting changes in transmitter production and in the sensitivity of postsynaptic receptors (Nutt & Glue, 1993). The shocks also stimulate the growth of new neurons in the brain, which some believe may contribute to their antidepressant effect (Jacobs, 2004).
The most frequent side effect of ECT is memory loss, both retrograde amnesia (being unable to remember events immediately before the event) and anterograde amnesia (being unable to form new memories following the event) (Merkl et al., 2009). In most cases, the memory loss clears up within a few months of the treatment (Squire & Slater, 1983).
Psychosurgery, Deep Brain Stimulation, and Transcranial Magnetic Stimulation
A treatment of last resort today is psychosurgery, which involves surgically cutting or producing lesions in portions of the brain to relieve a mental disorder. From the late 1930s into the early 1950s, tens of thousands of men and women were subjected to an operation called prefrontal lobotomy, in which the anterior (front) portions of the frontal lobes were surgically separated from the rest of the brain. Individuals with severe cases of schizophrenia, bipolar disorder, depression, obsessive-compulsive disorder, and pathological violence were subjected to the operation. Prefrontal lobotomy was so highly regarded that in 1949 the Portuguese neurologist who developed the technique, Egas Moniz, was awarded the Nobel Prize.
By the mid-1950s, however, prefrontal lobotomies had gone out of style, partly because newly developed drug treatments offered an alternative and partly because of mounting evidence that, although lobotomy relieved people of their incapacitating emotions, it left them severely incapacitated in new ways (Valenstein, 1986). As we have mentioned numerous times in earlier chapters, the prefrontal lobes are a critical part of the brain’s circuitry for executive functions—the ability to integrate plans with action—and lobotomized patients showed lifelong inabilities to make plans and behave according to them. As a consequence, they needed constant care.
672
8
How are modern, refined forms of psychosurgery sometimes used today in the treatment of obsessive-compulsive disorder?
Refined versions of psychosurgery were developed in the 1960s and continue to be used in rare cases today. The new procedures involve destruction of very small areas of the brain by applying radiofrequency current through fine wire electrodes implanted temporarily into the brain. Today this procedure is used primarily for treatment of highly incapacitating cases of obsessive-compulsive disorder that have proven, over many years, to be untreatable by any other means.
Obsessive-compulsive disorder is often associated with abnormal amounts of activity in a neural circuit that is involved in converting conscious thoughts into actions. This circuit includes a portion of the prefrontal cortex, a portion of the limbic system called the cingulum, and parts of the basal ganglia. Surgical destruction either of a portion of the cingulum or of a specific neural pathway that enters the basal ganglia reduces or abolishes obsessive-compulsive symptoms in about 50 percent of people who could not be successfully treated in any other way (Mashour et al., 2005). These procedures produce quite serious side effects in some patients, however, including confusion, weight gain, depression, and, in rare cases, epilepsy (Mashour et al., 2005; Trimble, 1996).
9
What is the advantage of deep brain stimulation over current forms of psychosurgery?
Since the mid 1990s, a number of brain surgeons have been experimenting with a new, safer procedure, called deep brain stimulation, for treating intractable cases of obsessive-compulsive disorder (Greenberg et al., 2006; Larson, 2008) and depression (Mayberg et al., 2005). In this procedure a hair-thin wire electrode is implanted permanently into the brain—usually in the cingulum or in a portion of the basal ganglia for patients being treated for obsessive-compulsive disorder. The electrode can be activated in order to electrically stimulate, rather than destroy, the neurons lying near it. High-frequency but low-intensity stimulation through the electrode is believed to desynchronize and disrupt ongoing neural activity and in that way to have an effect comparable to producing a lesion. This effect, unlike that of a lesion, can be reversed just by turning off the electrical current. Trials with deep brain stimulation suggest that it may be as effective as psychosurgery, without the negative side effects (Husted & Shapira, 2004; Larson, 2008).
673
In Chapter 5 we introduced the technique of transcranial magnetic stimulation as a form of mapping the brain’s functions (p. 160). In this method, a technician sends a pulse of electricity through a small copper coil, held just above a person’s head. The magnetic field passes through the scalp and skull and induces an electric current in the neurons immediately below the coil. When focused on the prefrontal cortex (see p. 175 in Chapter 5), changes in the activity of neurons reduces depression in some patients when it is administered daily over 2 to 4 weeks (Fox et al., 2012; Rosenberg et al., 2011).
SECTION REVIEW
Biological treatments target the brain physically in order to alleviate mental disorders.
Drugs
- Antipsychotic drugs treat psychotic symptoms, but do not cure people. All such drugs decrease the effectiveness of the neurotransmitter dopamine, and the newer (atypical) drugs also affect other neurotransmitters. All have unpleasant side effects, some quite serious.
- Antianxiety drugs, in a chemical class called benzodiazepines, are used mainly for generalized anxiety disorder and panic disorder. They increase inhibitory activity in the brain, thus reducing excitability; but they are addictive, have potentially harmful side effects, and cause unpleasant withdrawal symptoms.
- Antidepressant drugs include tricyclics and selective serotonin reuptake inhibitors (SSRIs); the former prolong the action of serotonin and norepinephrine in the brain, while the latter affect only serotonin. They are equally effective in treating depression, but SSRIs have milder side effects and are therefore more often prescribed. The SSRIs are also used to treat anxiety disorders.
- Studies that break down patients’ improvement after taking an antidepressant drug into three categories—spontaneous recovery, placebo effect, and drug effect—reveal that much, if not most, of the improvement is due to the placebo effect. Hope, provided by the sense of being treated, may be the principal ingredient of any treatment for depression.
Other Biological Treatments
- In electroconvulsive therapy (ECT), used to treat depression not helped by other means, electrical current is applied to the skull to induce brain seizures. It is quite safe and effective but causes some loss of recent memories.
- Prefrontal lobotomies, once common, are no longer performed. Today psychosurgery involving small, localized lesions is used occasionally for incapacitating obsessive-compulsive disorder. It is often effective but can produce harmful side effects.
- Deep brain stimulation, a possible alternative to psychosurgery, uses electrical current to disrupt activity rather than destroy tissue at specific brain locations.
- Transcranial magnetic stimulation sends a pulse of electricity through a coil held just above a person’s head, inducing an electric current in the neurons immediately below the coil, and can be effective in treating depression.