3.2 Inheritance of Behavioral Traits
Variation in genes contributes to the variation in behavior that one sees in any animal species. Some behavioral characteristics are inherited in accordance with the same pattern that Mendel observed in plants, indicative of control by a single pair of genes. Most behavioral characteristics, however, depend on many genes. In this section, we shall look first at two examples of single-gene traits and then at traits that are affected by many genes.
Examples of Single-Gene (Mendelian) Inheritance
Mendelian Inheritance of Fearfulness in Dogs
9
How did Scott and Fuller show that the difference in fearfulness between cocker spaniels and basenji hounds is controlled by a single gene locus, with the “fear” allele dominant over the “nonfear” allele?
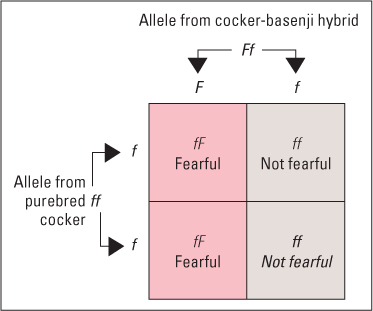
One of the first demonstrations of single-gene control of a behavioral trait in dogs was published half a century ago. In pioneering research on the role of genes in behavior, John Paul Scott and John Fuller (1965) studied the behavior of basenji hounds, cocker spaniels, and their mixed-breed offspring. Basenjis are timid dogs, showing fear of people until they have received much gentle handling. Cockers, in contrast, show little fear under normal rearing conditions. In a standard test with 5-week-old puppies, Scott and Fuller found that all the basenji puppies yelped and/or ran away when approached by a strange person, whereas only a few of the cocker puppies showed these reactions. When cockers and basenjis were crossbred, the offspring (F1 hybrids) were like basenjis in this test: All showed signs of fear when approached. Since this was as true of hybrids raised by cocker mothers as of those raised by basenji mothers, Scott and Fuller concluded that the effect stemmed from the hybrid dogs’ genes and not from anything they learned from their mothers.
The fact that the F1 hybrids were as fearful as the purebred basenjis suggested to Scott and Fuller that the difference in fearfulness between the two purebred strains might be controlled by a single gene locus, with the allele promoting fear dominant over that promoting confidence. If this were so, then mating F1 hybrids with each other should produce a group of offspring (F2 generation) in which three-fourths would show basenji-like fear and one-fourth would show cocker-like confidence—the same ratios that Mendel had found with seed texture in pea plants. Scott and Fuller did this experiment and, indeed, found ratios very close to those predicted. As additional evidence, they also mated F1 hybrids with purebred cockers. About half the offspring of those backcrosses were basenji-like in fear, and the other half were cocker-like in confidence—just as can be expected if the “fear” allele is dominant over the “nonfear” allele (see Figure 3.7).
10
Why would it be a mistake to conclude, from Scott and Fuller’s work, that fear in dogs is caused just by one gene or that it is caused just by genes and not by the environment?
65
Be careful not to misinterpret this finding. It concerns a difference between two breeds of dogs in certain behavioral tests. It would not be reasonable to conclude that fear in all its various forms is controlled by a single gene. Many different genes must contribute to building the complex neural structure needed to experience fear and express it in behavior. Scott and Fuller’s work demonstrates only that the difference between cocker spaniels and basenji hounds in a particular test of fear is controlled by a single gene. Recognize also that their studies do not diminish the role of environmental influences. Scott and Fuller could detect the effect of a specific gene pair because they raised all the dogs in similar environments. In other research, Scott (1963) showed that any puppy isolated from people for the first 4 months of life will be fearful of humans. Had Scott and Fuller isolated the cockers from all human contact and given the basenjis lots of kind handling before the behavioral test, they might well have found the cockers to be more fearful than the basenjis, despite the genetic predispositions toward the opposite behavior.
Mendelian Inheritance and Expression of Genetic Disorders
11
How do genes and the environment interact to affect individuals with PKU?
Most of the behaviorally relevant traits in humans that derive from alteration at a single gene locus are brain disorders caused by relatively rare, mutant, malfunctioning genes that are passed from generation to generation. Some single-gene metabolic disorders are passed in this way as well. For example, in phenylketonuria, or PKU, infants inherit two recessive genes involved in the processing of the amino acid phenylalanine. The presence of these genes causes the amino acid to accumulate in the brain, resulting in intellectual impairment. However, PKU only has its detrimental effects if the person consumes foods that contain phenylalanine. (Phenylalanine is found in many foods and is a principal ingredient in some artificial sweeteners made with Aspartame®.) Newborns are routinely screened for the ability to process phenylalanine, and when babies who have the PKU genes are placed on a phenylalanine-free diet, they develop normally. Genes themselves, then, do not “cause” the symptoms of PKU—excessive phenylalanine in the diet does. However, the inability to process phenylalanine is “caused” by defective genes. Thus, even in this prototypical case of a genetic disease, genes and environment clearly interact (Widaman, 2009).
Polygenic Characteristics and Selective Breeding
12
How does the distribution of scores for a polygenic trait differ from that usually obtained for a single-gene trait?
Characteristics that derive from variation at a single gene locus are typically categorical in nature. That is, they are characteristics that sharply differentiate one group from another. Peas are either round or wrinkled; mixed-breed basenji-cockers differ so sharply from one another in fearfulness that they can be categorized into two distinct groups; newborn babies either have or do not have PKU (none of them “sort of have it”).
66
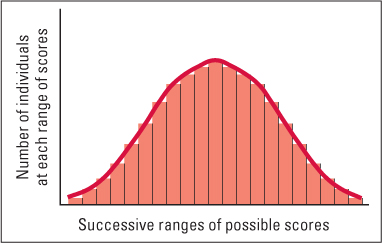
But most anatomical and behavioral differences among individuals of any species are measurable in degree, not type. They are continuous rather than categorical. That is, the measures taken from individuals do not fall into two or more distinct groups but can lie anywhere within the observed range of scores. Most often, the set of scores obtained on such measures approximate a normal distribution, meaning that most scores fall near the middle of the range and the frequency tapers off toward the two extremes (see Figure 3.8). Measures of aggressiveness in mice, of maze learning in rats, and of conscientiousness in people are just a few of the behavioral measures that are consistently found to fit a normal distribution.
Characteristics that vary in a continuous way are generally affected by many genes and are therefore called polygenic characteristics (the prefix poly- means “many”). Of course, these traits are also influenced by variation in the environment, so the variability observed in a graph such as Figure 3.8 results from a combination of genetic differences at many gene loci and environmental differences. In animals, it is possible to study the role of genes in polygenic traits through the procedure of selective breeding.
Selective Breeding for Behavioral Characteristics in Animals
13
How are the characteristics of animals shaped through selective breeding?
To the degree that individuals within a species differ in any measurable characteristic because of differences in their genes, that characteristic can be modified over successive generations through selective breeding. This procedure involves the mating of individuals that lie toward the same extreme on the measure in question. For single-gene characteristics the effects of selective breeding are immediate, but for polygenic characteristics the effects are gradual and cumulative over generations.

The basic procedure of selective breeding is by no means new. For thousands of years before a formal science of genetics existed, plant and animal breeders used selective breeding to produce new and better strains of every sort of domesticated species. Grains were bred for plumper seeds; cows, for docility and greater milk production; horses, along separate lines for working and racing; canaries, for their song; and dogs, along dozens of different lines for such varied purposes as following a trail, herding sheep (running around them instead of at them), and being gentle playmates for children. The procedure in every case was essentially the same: The members of each generation that best approximated the desired type were mated to produce the next generation, resulting in a continuous genetic molding toward the varieties we see today.
Under controlled laboratory conditions, researchers have used selective breeding to produce many behaviorally specialized strains of animals, usually for the purpose of understanding better the biological foundations of the behaviors in question. Fruit flies have been bred to move instinctively either toward or away from a source of light, mice to be either more or less inclined to fight, rats to either prefer or not prefer alcohol over water, and foxes to be either highly aggressive or extraordinarily docile and friendly toward humans (Crabbe et al., 1999a; Kukekova et al., 2008; Wimer & Wimer, 1985). That selective breeding can influence essentially any behavioral trait should come as no surprise. It follows logically from the fact that all behaviors depend on particular sensory, motor, and neural structures, all of which are built from proteins whose production depends on genes.
67
Selective Breeding for Maze Learning: Tryon’s Classic Research
The first long-term, systematic study of selective breeding in psychology was begun in the 1920s by Robert Tryon (1942). Tryon wanted to demonstrate that a type of behavior frequently studied by psychologists could be strongly influenced by variation in genes.
14
How did Tryon produce “maze bright” and “maze dull” strains of rats? How did he show that the difference was the result of genes, not rearing?
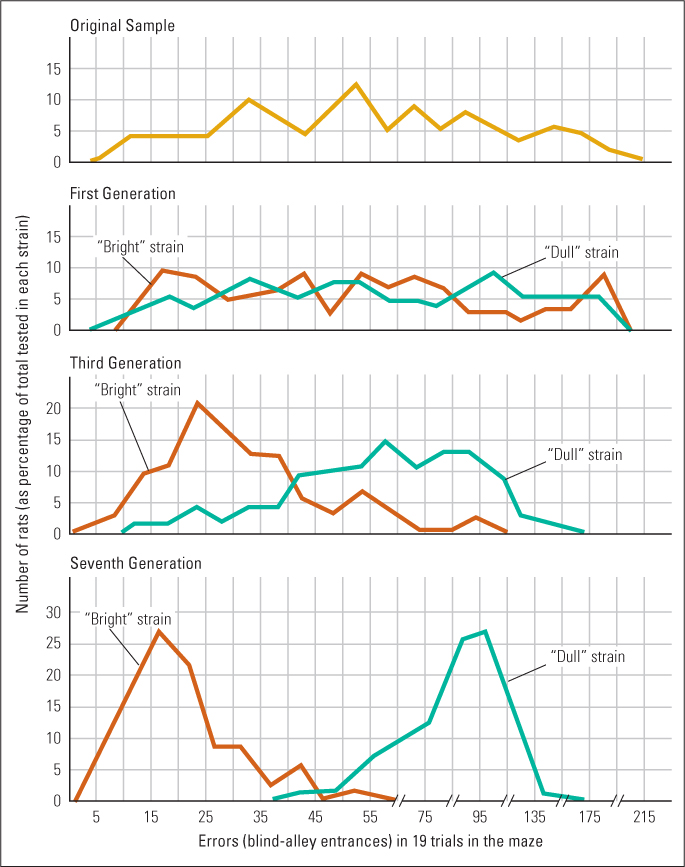
Tryon began by testing a genetically diverse group of rats for their ability to learn a particular maze. Then he mated the males and females that had made the fewest errors in the maze to begin what he called the “maze bright” strain and those that had made the most errors to begin the “maze dull” strain. When the offspring of succeeding generations reached adulthood, he tested them in the same maze and mated the best-performing members of the bright strain, and the worst-performing members of the dull strain, to continue the two lines.
Some of his results are shown in Figure 3.9. As you can see, with each generation the two strains became increasingly distinct, until by the seventh there was almost no overlap between them. Almost all seventh-generation bright rats made fewer errors in the maze than even the best dull rats. To control for the possibility that the offspring were somehow learning to be bright or dull from their mothers, Tryon cross-fostered the rats so that some of the offspring from each strain were raised by mothers in the other strain. He found that rats in the bright strain were equally good in the maze, and those in the dull strain equally poor, regardless of which mothers raised them.
68
15
Why is the strain difference produced by Tryon not properly characterized in terms of “brightness” or “dullness”?
Once a strain has been bred to show some behavioral characteristic, the question arises as to what other behavioral or physiological changes accompany it. Tryon referred to his two strains of rats as “bright” and “dull,” but all he had measured was their performance in a particular type of maze. Performance in the maze no doubt depended on many sensory, motor, motivational, and learning processes, and specific changes in any of them could in theory have mediated the effects that Tryon observed. In theory, Tryon’s “dull” rats could simply have been those that had less acute vision, or were less interested in the variety of food used as a reward, or were more interested in exploring the maze’s blind alleys.
In later studies, another researcher found that Tryon’s “dull” rats were as good as the “bright” ones, and sometimes even better, at other learning tasks (Searle, 1949). We do not know what underlying abilities or dispositions changed in Tryon’s two strains of rats to produce their difference in maze performance, but the change was apparently not one of general learning ability. This problem still occurs in modern behavioral genetics research, in which new strains of animals (usually mice) are created by adding, deleting, or modifying known genes using sophisticated genetic-engineering methods. The behavioral differences between two strains found in one laboratory often fail to occur in another laboratory, apparently because of subtle differences in the way the animals are housed or tested (Cabib et al., 2000; Crabbe et al., 1999b).
Polygenic Behavioral Characteristics in Humans
Most of the measures of human traits that interest psychologists—such as scores on personality tests—are continuous and normally distributed and are affected by many genes as well as by environmental variables. Some psychologists are interested in the degree to which the differences in such scores, for a particular group of people, are the result of differences in their genes or differences in their environmental experiences. Of course, psychologists can’t perform selective breeding studies with humans, but they have developed other methods to estimate that degree. Those methods involve comparing the average difference in test scores for people who are closely related to one another with that for people who are less closely related, using people who are chosen in such a way that the environments of the closely related people are no more similar to one another than are those of the less closely related people. Comparisons of identical twins with fraternal twins, and of biologically related siblings with adoptive siblings, have proven particularly useful. In later chapters you will read about such methods as they apply to a variety of psychological topics including intelligence tests, personality tests, and predisposition to various mental disorders.
A Few Words About Epigenetics
16
How might a better understanding of epigenetics change the way we view genetic inheritance?
It was not long after the publication of the first “complete” drafts of the human genome in February 2001 (International Human Genome Sequencing Consortium, 2001; Venter et al., 2001) that biologists realized that genes are only part of the story. Identical twins, and even cloned animals, are different from one another at birth, and these differences cannot be attributed to genes. Instead, they have been attributed to epigenetic effects. The field of epigenetics examines “gene-regulating activity that doesn’t involve changes to the DNA code and that can persist through one or more generations” (Pennisi, 2001, p. 1064). We inherit from our parents not only DNA but also a variety of chemical markers that regulate genes, turning them on at certain times and off at others and determining how much protein they produce. Recall that every cell in your body has the same DNA, but only some of it is active at any one time. Although each cell possesses the genetic information to grow an eye, for example, eyes do not grow from your liver or on your elbows. Epigenetic mechanisms are responsible for this.
69
The best understood mechanism for epigenetic effects is that of DNA methylation. The DNA of all plants and vertebrates and many invertebrates has chemicals from the methyl group (written CH3 by chemists) attached to some of its nucleic acids. Methylation does not alter the protein that a gene will produce, but rather influences whether the genes will produce the protein at all. Most highly methylated genes do not produce their proteins, that is, they are “shut off” (Jablonka & Lamb, 2005).
Processes of DNA methylation seem highly regulated and similar across individuals. For example, in early development, genes that govern the building of an eye become methylated and “turn off” in all tissues except those that will eventually develop into eyes. However, DNA methylation can also be influenced by experience; indeed, it seems to be the primary mechanism by which experience modifies gene action and thus behavior. Amazingly, some of the epigenetic markers created by methylation can be inherited, along with the behavior they influence, as shown in the following example. Mother rats groom their infant pups, mostly by licking them. Pups that are licked a lot (that is, who have “high-licking mothers”) grow up to be less vulnerable to stress than pups with “low-licking mothers.” When the female pups mature and become mothers themselves, they show the same licking pattern as their mothers. This is true even when the pups are cross-fostered (that is, when a pup born to a low-licking mother is raised by a high-licking mother or vice versa). As adults, they show the pattern of their foster mother, not their genetic mother, and this pattern continues for at least several generations (Francis et al., 1999; Meaney, 2001, 2010). Michael Meaney and his colleagues documented the biochemical mechanisms responsible for these transgenerational effects, showing how early experience can alter behavior and be transmitted to future generations, all without any changes in the genes themselves.
Something similar seems to happen in humans. For example, excessive childhood stress in the form of abuse or neglect is associated with poor mental health in later life, including personality disorders, depression, anxiety disorders, and substance abuse (Cicchetti & Toth, 2006). The hormone cortisol is associated with such stress, and complex interactions between cortisol, brain activity, and experience over the course of development are related to how people respond to stress (Carpenter et al., 2004; Lee et al., 2005). Discerning the chemical causes of behavior is difficult in humans (few people allow the chemicals in their brains to be assayed while they are still alive). However, research with nonhuman animals suggests that the way the human brain “learns” to react to stress through the production and processing of the hormone cortisol is likely governed by epigenetic mechanisms (Carey, 2012).
Although we know of no studies showing the transmission of behavior across generations in humans via epigenetic mechanisms, there is such evidence in the realm of physical development from survivors of the Dutch Hunger Winter. During World War II, parts of the Netherlands experienced extreme famine. For women who were pregnant during this time, not only they, but their unborn children, were severely deprived of calories. The Dutch government followed these children, who were conceived during the famine, as well as the next generation. When women suffered malnutrition during their first 3 months of pregnancy, their babies were born with normal weight but were at high risk for obesity as adults. Moreover, when these female babies became mothers themselves, their offspring were heavier than average. That is, experiences of the grandmother while pregnant influenced the development of her grandchildren (Lumey, 1998). A similar phenomenon has been reported on the effects of malnutrition on the subsequent growth rate and health (susceptibility to cardiovascular disease) for a sample of Swedish men (Kaati et al., 2002).
70
The field of epigenetics is just coming into its own, but new discoveries promise to shed light on the biological basis of inheritance, including ways in which experiences during a person’s lifetime might influence the phenotype of his or her grandchildren, as well as defining a bit more clearly the nature of geneenvironment interactions.
SECTION REVIEW
Hereditary effects on behavioral traits can involve just one gene but usually involve many.
Single-Gene Traits
- Single-gene traits (controlled by one pair of genes) are categorical (all or none) in nature.
- Mendelian patterns of inheritance indicate single-gene control.
- Examples are breed differences in fearfulness in dogs and the human hereditary disorder phenylketonuria (PKU).
Polygenic Traits
- Polygenic traits (influenced by many gene pairs) are continuous (present in varying degrees) and often fit a normal distribution.
- Through selective breeding, a trait can be strengthened or weakened gradually over generations.
- Examples include Tryon’s breeding of rats for maze ability and the Russian program of breeding foxes for tameness.
Epigenetics
- Experiences of the individual can cause biochemical changes that affect the activation of genes, and these activation “signals” can be transmitted to children and grandchildren without any changes in the genes themselves.