Concept 19.1: Life Consists of Three Domains That Share a Common Ancestor
You may think that you have little in common with a bacterium. But all multicellular eukaryotes—including you—share many attributes with bacteria and archaea, together called prokaryotes. For example, all organisms, whether eukaryotes or prokaryotes,
- have cell membranes and ribosomes (see Chapter 4).
- have a common set of metabolic pathways, such as glycolysis (see Chapter 6).
- replicate DNA semiconservatively (see Chapter 9).
- use DNA as the genetic material to encode proteins, and use a similar genetic code to produce those proteins by transcription and translation (see Chapter 10).
These shared features support the conclusion that all living organisms are related. If life had multiple origins, there would be little reason to expect all organisms to use overwhelmingly similar genetic codes or to share structures as unique as ribosomes. Furthermore, similarities in the DNA sequences of genes that are shared by all organisms confirm the monophyly of life.
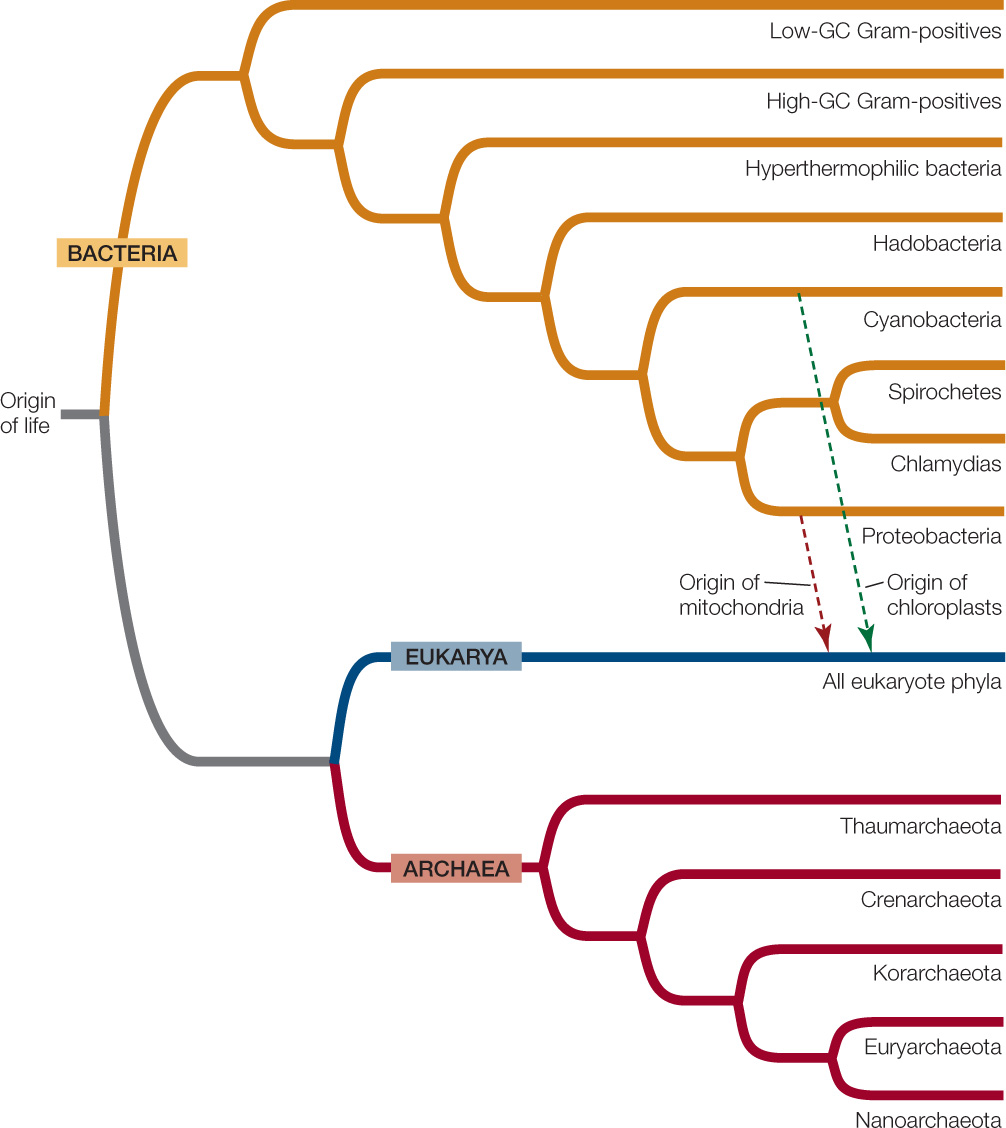
Despite these commonalities, major differences have also evolved across the diversity genetic of life. Based on the deepest divisions of phylogenetic relationships, many biologists now recognize three domains (primary divisions) of life, two prokaryotic and one eukaryotic (FIGURE 19.1).

Go to ANIMATED TUTORIAL 19.1 The Evolution of the Three Domains
PoL2e.com/at19.1
All prokaryotic organisms are unicellular, although they may form large coordinated colonies or biofilms consisting of many individuals. The domain Eukarya, by contrast, encompasses both unicellular and multicellular life forms. As was described in Chapter 4, prokaryotic cells differ from eukaryotic cells in some important ways:
- Prokaryotic cells do not divide by mitosis. Instead, after replicating their DNA, prokaryotic cells divide by their own method, binary fission (see Concept 7.2).
- The organization of the genetic material differs. The DNA of the prokaryotic cell is not organized within a membrane-enclosed nucleus. DNA molecules in prokaryotes are often circular. Many (but not all) prokaryotes have only one main chromosome and are effectively haploid, although many have additional smaller DNA molecules, called plasmids (see Concept 12.2).
- Prokaryotes lack most of the membrane-enclosed cytoplasmic organelles—mitochondria, Golgi apparatus, and others—that are found in most eukaryotes. However, the cytoplasm of a prokaryotic cell may contain a variety of infoldings of the cell membrane and photosynthetic membrane systems not found in eukaryotes.
Although the study and classification of eukaryotic organisms goes back centuries, much of our knowledge of the evolutionarily ancient prokaryotic domains is quite recent. Not until the final quarter of the twentieth century did advances in molecular genetics and biochemistry reach a point that enabled research that revealed deep-seated distinctions between the domains Bacteria and Archaea.
380
The two prokaryotic domains differ in significant ways
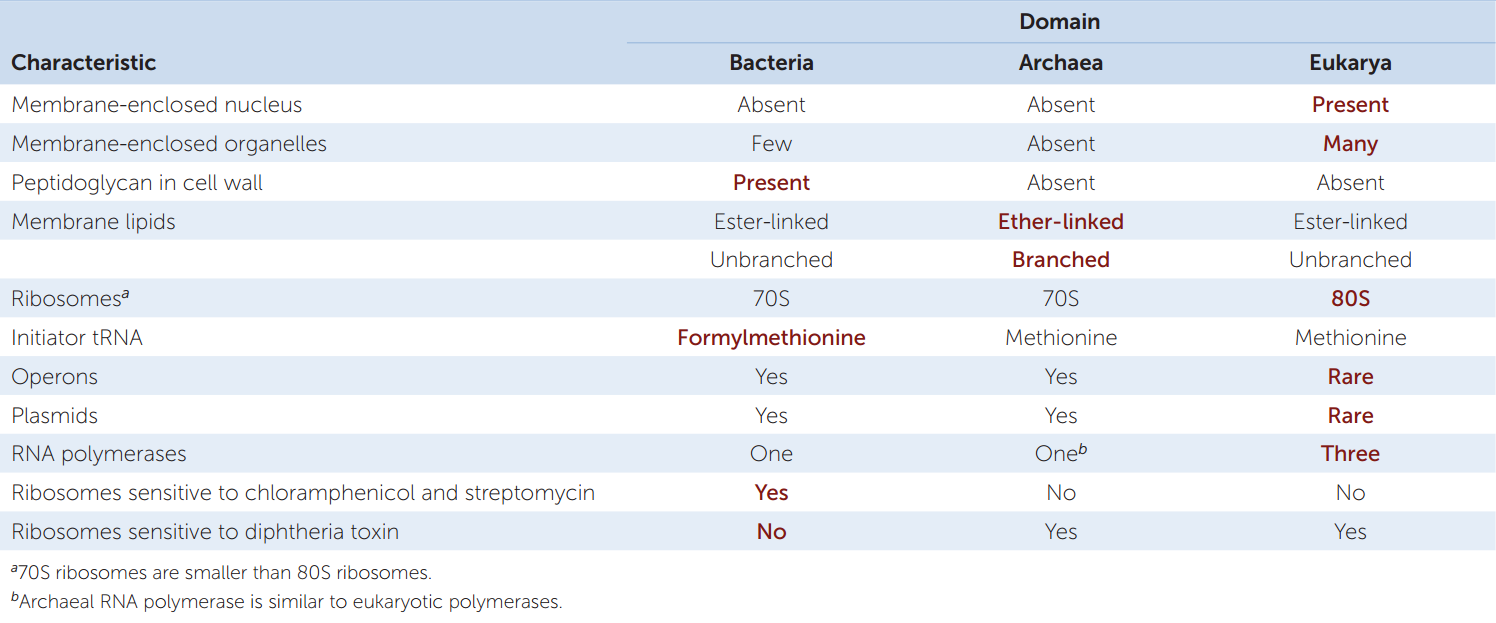
A glance at TABLE 19.1 will show you that there are major differences (most of which cannot be seen even under an electron microscope) between the two prokaryotic domains. In some ways archaea are more like eukaryotes; in other ways they are more like bacteria. (Note that we use lowercase when referring to the members of these domains and uppercase when referring to the domains themselves.) The basic unit of an archaeon (the term for a single archaeal organism) or bacterium (a single bacterial organism) is the prokaryotic cell. Each single-celled organism contains a full complement of genetic and protein-synthesizing systems, including DNA, RNA, and all the enzymes needed to transcribe and translate the genetic information into proteins. The prokaryotic cell also contains at least one system for generating the ATP it needs.
Genetic studies clearly indicate that all three domains had a single common ancestor. Across a major portion of their genome, eukaryotes share a more recent common ancestor with Archaea than they do with Bacteria (see Figure 19.1). However, the mitochondria of eukaryotes (as well as the chloroplasts of photosynthetic eukaryotes, such as plants) originated through endosymbiosis with a bacterium. Some biologists prefer to view the origin of eukaryotes as a fusion of two equal partners (one ancestor that was related to modern archaea, and another that was more closely related to modern bacteria). Others view the divergence of the early eukaryotes from the archaea as a separate and earlier event than the later endosymbioses. In either case, some eukaryote genes are most closely related to those of archaea, whereas others are most closely related to those of bacteria. The tree of life therefore contains some merging of lineages as well as the predominant divergence of lineages.
LINK
The origin of mitochondria and chloroplasts by endosymbiosis is described in Concept 20.1
Biologists estimate that the last common ancestor of the three domains lived about 3 billion years ago. We can deduce that it had DNA as its genetic material, and that its machinery for transcription and translation produced RNAs and proteins, respectively. This ancestor likely had a circular chromosome. Archaea, Bacteria, and Eukarya are all the products of billions of years of mutation, natural selection, and genetic drift, and they are all well adapted to their present-day environments. The earliest prokaryote fossils, which date back at least 3.5 billion years, indicate that there was considerable diversity among the prokaryotes even during those earliest days of life.
The small size of prokaryotes has hindered our study of their evolutionary relationships
Until about 300 years ago, nobody had even seen an individual prokaryote. Most prokaryotes remained invisible to humans until the invention of the first simple microscope. Prokaryotes are so small, however, that even the best light microscopes don’t reveal much about them. It took advanced microscopic equipment and modern molecular techniques to open up the microbial world. (Microscopic organisms—both prokaryotes and eukaryotes—are often collectively referred to as “microbes.”)
Before DNA sequencing became practical, taxonomists based prokaryote classification on observable characters such as shape, color, motility, nutritional requirements, and sensitivity to antibiotics. One of the characters most widely used to classify prokaryotes is the structure of their cell walls.
The cell walls of almost all bacteria contain peptidoglycan, a polymer that produces a meshlike structure around the cell. Peptidoglycan is a substance unique to bacteria. The absence of peptidoglycan from the cell walls of archaea is a key difference between the two prokaryotic domains. Peptidoglycan is also an excellent target for combating pathogenic (disease-causing) bacteria because it has no counterpart in eukaryotic cells. Antibiotics such as penicillin and ampicillin, as well as other agents that specifically interfere with the synthesis of peptidoglycan-containing cell walls, tend to have little, if any, effect on the cells of humans and other eukaryotes.
381
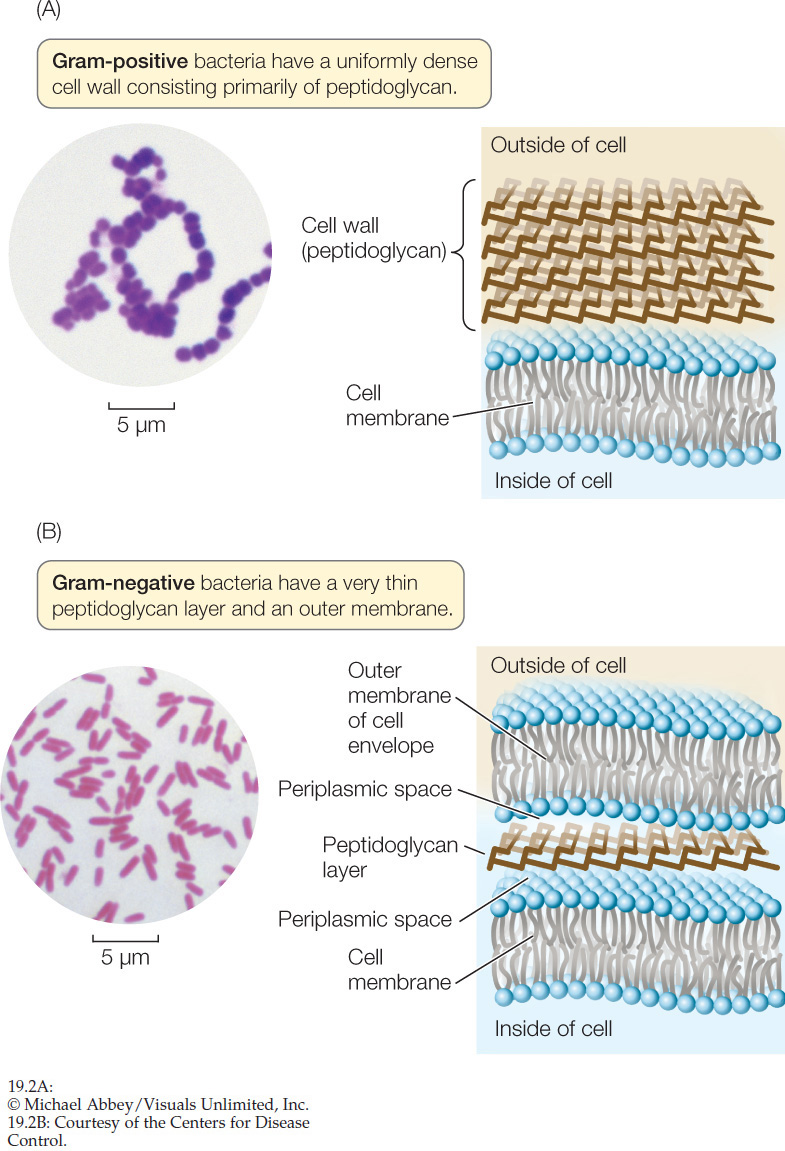
The Gram stain is a laboratory technique that can be used to classify most types of bacteria into two distinct groups, Gram-positive and Gram-negative. A smear of bacterial cells on a microscope slide is soaked in a violet dye and treated with iodine. Next it is washed with alcohol and counterstained with a red dye (safranin). Gram-positive bacteria retain the violet dye and appear blue to purple (FIGURE 19.2A). The alcohol washes the violet stain out of Gram-negative bacteria, which then pick up the safranin counterstain and appear pink to red (FIGURE 19.2B). For most bacteria, the effect of the Gram stain is determined by the chemical structure of the cell wall:
- A Gram-negative cell wall usually has a thin peptidoglycan layer, which is surrounded by a second, outer membrane quite distinct in chemical makeup from the cell membrane (see Figure 19.2B). The space between the cell membrane and the outer membrane (known as the periplasmic space) contains proteins that are important in digesting some materials, transporting others, and detecting chemical gradients in the environment.
- A Gram-positive cell wall usually has about five times as much peptidoglycan as a Gram-negative cell wall. Its thick peptidoglycan layer is a meshwork that may serve some of the same purposes as the periplasmic space of the Gram-negative cell envelope (cell wall plus outer membrane).
Go to ACTIVITY 19.1 Gram Stain and Bacteria
PoL2e.com/ac19.1
Shape is another phenotypic characteristic that is useful for the basic identification of bacteria. Three major shapes are common—spheres, rods, and spiral forms (FIGURE 19.3). Many bacterial names are based on these shapes. A spherical bacterium is called a coccus (plural cocci). Cocci may live singly or may associate in two- or three-dimensional arrays as chains, plates, blocks, or clusters of cells. A rod-shaped bacterium is called a bacillus (plural bacilli). A spiral bacterium (shaped like a corkscrew) is called a spirillum (plural spirilla). Bacilli and spirilla may be single, form chains, or gather in regular clusters. Among the other bacterial shapes are long filaments and branched filaments.
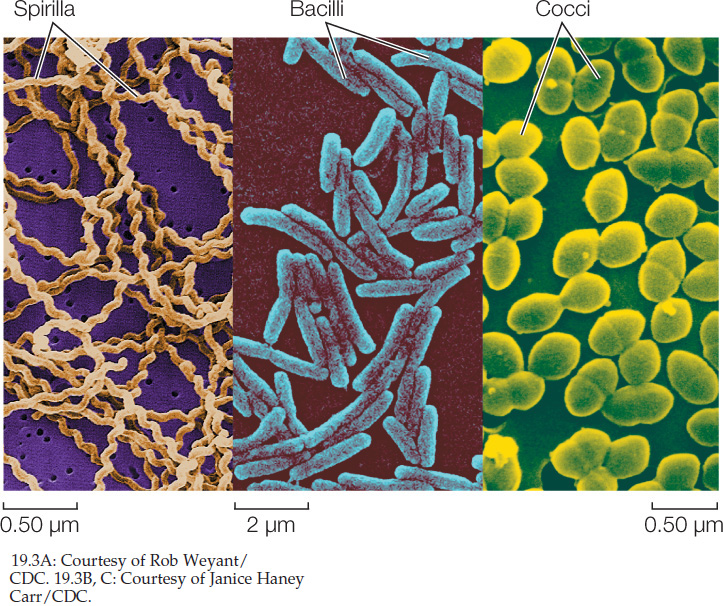
Less is known about the shapes of archaea because many of these organisms have never been seen. Many archaea are known only from samples of DNA from the environment. However, the species whose morphologies are known include cocci, bacilli, and even triangular and square-shaped species. Some flattened species grow on surfaces, arranged like sheets of postage stamps.
The nucleotide sequences of prokaryotes reveal their evolutionary relationships
Analyses of the nucleotide sequences of ribosomal RNA (rRNA) genes provided the first comprehensive evidence of evolutionary relationships among prokaryotes. For several reasons, rRNA is particularly useful for phylogenetic studies of living organisms:
382
- rRNA was present in the common ancestor of all life and is therefore evolutionarily ancient.
- No free-living organism lacks rRNA, so rRNA genes can be compared throughout the tree of life.
- rRNA plays a critical role in translation in all organisms, so lateral transfer of rRNA genes among distantly related species is unlikely.
- rRNA has evolved slowly enough that gene sequences from even distantly related species can be aligned and analyzed.
Comparisons of rRNA genes from a great many organisms have revealed the probable phylogenetic relationships throughout the tree of life. Databases such as GenBank contain rRNA gene sequences from hundreds of thousands of species—more than any other type of gene sequence.
Although studies of rRNA genes reveal much about the evolutionary relationships of prokaryotes, they don’t always reveal the entire evolutionary history of these organisms. In some groups of prokaryotes, analyses of multiple gene sequences have suggested several different phylogenetic patterns. How could such differences among different gene sequences arise? Studies of whole prokaryotic genomes have revealed that even distantly related prokaryotes sometimes exchange genetic material.
Lateral gene transfer can lead to discordant gene trees
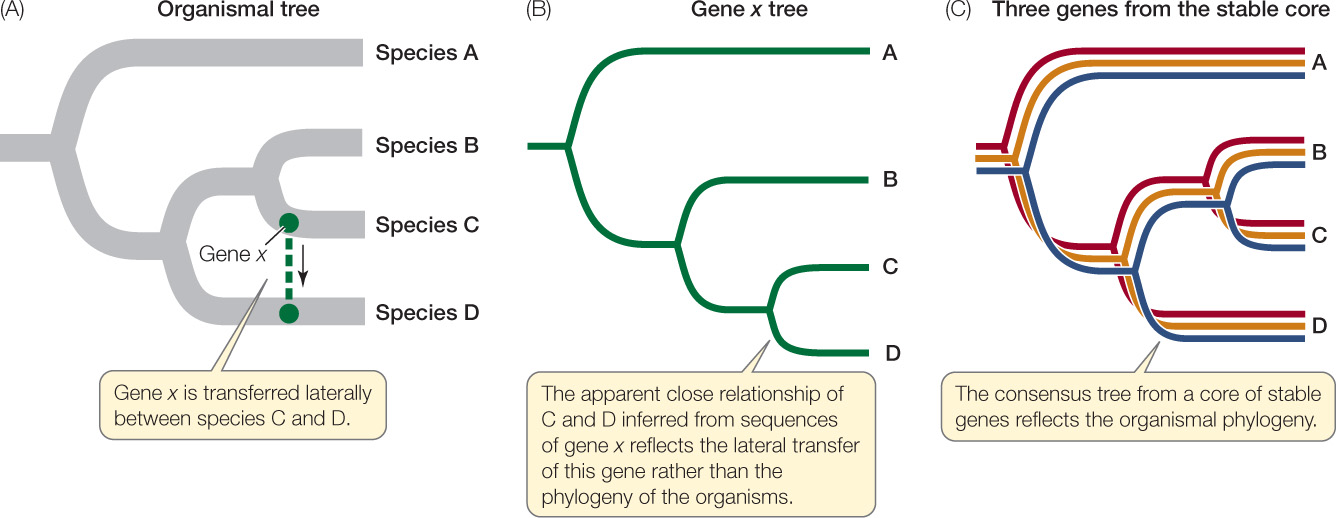
As noted earlier, prokaryotes reproduce by binary fission. If we could follow these divisions back through evolutionary time, we would be tracing the path of the complete tree of life for bacteria and archaea. This underlying tree of relationships (represented in highly abbreviated form in Appendix A) is called the organismal (or species) tree. Because whole genomes are replicated during binary fission, we would expect phylogenetic trees constructed from most gene sequences (see Chapter 16) to reflect these same relationships.
Even though binary fission is an asexual process, there are other processes—including transformation, conjugation, and transduction—that allow the exchange of genetic information between some prokaryotes without reproduction. Thus prokaryotes can exchange and recombine their DNA with that of other individuals (this is sex in the genetic sense of the word), but this genetic exchange is not directly linked to reproduction, as it is in most eukaryotes.
LINK
Prokaryote exchange of genetic material by transformation, conjugation, and transduction is described in Concept 8.4
From early in evolution to the present day, some genes have been moving “sideways” from one prokaryote species to another, a phenomenon known as lateral gene transfer. Lateral gene transfers are well documented, especially among closely related species; some have been documented even across the domains of life.
Consider, for example, the genome of Thermotoga maritima, a bacterium that can survive extremely high temperatures. In comparing the 1,869 gene sequences of T. maritima against sequences encoding the same proteins in other species, investigators found that some of this bacterium’s genes have their closest relationships not with the genes of other bacterial species, but with the genes of archaea that live in similar extreme environments.
When genes involved in lateral transfer events are sequenced and analyzed, the resulting individual gene trees will not match the organismal tree in every respect (FIGURE 19.4). The individual gene trees will vary because the history of lateral transfer events is different for each gene. Biologists can reconstruct the underlying organismal phylogeny by comparing multiple genes (to produce a consensus tree) or by concentrating on genes that are unlikely to be involved in lateral gene transfer events. For example, genes that are involved in fundamental cellular processes (such as the rRNA genes discussed above) are unlikely to be replaced by the same genes from other species because functional, locally adapted copies of these genes are already present.
383
What kinds of genes are most likely to be involved in lateral gene transfer? Genes that result in a new adaptation that confers higher fitness on a recipient species are most likely to be transferred repeatedly among species. For example, genes that produce antibiotic resistance are often transferred among bacterial species on plasmids, especially under the strong selection pressure such as that imposed by modern antibiotic medications. Improper or overly frequent use of antibiotics can select for resistant strains of bacteria that are much harder to treat. This selection for antibiotic resistance explains why informed physicians have become more careful in prescribing antibiotics.
It is debatable whether lateral gene transfer has seriously complicated our attempts to resolve the tree of prokaryotic life. Recent work suggests that it has not. Lateral gene transfer rarely creates problems at higher taxonomic levels, even though it may complicate our understanding of the relationships among individual species. Some species clearly obtain some of their genes from otherwise distantly related species, so evolutionary histories of individual genes may differ within a single organism. But it is now possible to make nucleotide sequence comparisons involving entire genomes, and these studies are revealing a stable core of crucial genes that are uncomplicated by lateral gene transfer. Gene trees based on this stable core more accurately reveal the organismal phylogeny (see Figure 19.4). The problem remains, however, that only a very small proportion of the prokaryotic world has been described and studied.
The great majority of prokaryote species have never been studied
Most prokaryotes have defied all attempts to grow them in pure culture, causing biologists to wonder how many species, and possibly even clades, we might be missing. A window onto this problem was opened with the introduction of a new way of examining nucleic acid sequences. When biologists are unable to work with the whole genome of a single prokaryote species, they can instead examine individual genes collected from a random sample of the environment (see Figure 12.6).
Norman Pace of the University of Colorado isolated individual rRNA gene sequences from extracts of environmental samples such as soil and seawater. Comparing such sequences with previously known ones revealed that an extraordinary number of the sequences were new, implying that they came from previously unrecognized species. Biologists have described only about 10,000 species of bacteria and only a few hundred species of archaea (see Figure 1.4). The results of Pace’s and similar studies suggest that there may be millions—perhaps hundreds of millions—of prokaryote species on Earth. Other biologists put the estimate much lower, arguing that the high dispersal ability of many bacterial species greatly reduces local endemism (i.e., the number of species restricted to a small geographic area). Only the magnitude of these estimates differs, however; all sides agree that we have just begun to uncover Earth’s bacterial and archaeal diversity.
CHECKpoint CONCEPT 19.1
- Why were all prokaryotes once considered members of a single clade, and what findings led to the establishment of Bacteria and Archaea as separate domains?
- How did biologists classify bacteria before it became possible to determine nucleotide sequences?
- Why are nucleotide sequences of rRNA genes particularly useful for evolutionary studies?
- How does lateral gene transfer complicate evolutionary studies?
Despite the challenges of reconstructing the phylogeny of prokaryotes, taxonomists are beginning to establish evolutionary classification systems for these organisms. With a full understanding that new information requires periodic revisions in these classifications, we will next apply a current system of classification to organize our survey of prokaryote diversity.