Concept 34.4: Sensory Processes Provide Information on an Animal’s External Environment and Internal Status
Animals require information on their external environments to function successfully. They need to perceive where they are going, and they need information to locate food, find mates, and avoid danger. Animals have evolved an enormous variety of sensory processes that enable them to gather information on their external environments. Imagine, for example, a male moth, an insect-eating bat, and a migrating bird moving across the landscape on a moonless, pitch-black night in late summer. The moth might be able to detect a female of his species, hundreds of yards away, by using sense organs that are extremely sensitive to odors that females release into the air. The bat might be able to zero in on him as he flies toward her by using echolocation. In this process, a bat emits bursts of vocal sound, at frequencies humans can’t hear, and senses the echoes of these sounds that bounce off the moth. The moth, in turn, might use its own sense of hearing to detect the bat’s vocalizations and take evasive action. Meanwhile the bird migrating overhead might use its eyes to locate a key star, which it can use as a navigational aid to fly south (see Concept 40.2).
Sensory processes are equally important for providing animals with information on their internal status. For instance, when we discussed breathing (see Chapter 31), we emphasized that the partial pressures of O2 and CO2 in an animal’s blood are important indicators of whether it is breathing properly. Sensory processes have evolved that enable animals to measure their internal O2 and CO2 partial pressures so that they can adjust their rate of breathing. Other internal sensory processes provide animals with information on the amount of tension produced by their muscles during contraction. This information can be used to adjust stimulation of the muscles by the nervous system so that the tension is neither too high nor too low.
Sensory receptor cells transform stimuli into electric signals
Sensory processes depend on sensory receptor cells, which are cells, typically neurons, specialized to transform the energy of a stimulus—called stimulus energy—into an electric signal. Energy transduction is the process of changing one type of energy into another. A sensory receptor cell is said to carry out sensory transduction when it produces an electric signal from stimulus energy.
The electric signal that is produced then directly or indirectly causes action potentials to be generated in a neuron. The action potentials—which encode the initial stimulus—convey the sensory information to the brain or other integrative parts of the nervous system for processing and interpretation. In this way, the animal acquires a sensory perception of its external environment and its internal status.
Sensory receptor cells are highly specific in the stimuli to which they ordinarily respond. A receptor cell that responds to vibration does not respond to light, and vice versa. A cell that responds to sweet tastes responds to neither vibration nor light.
Some stimuli—such as light, heat, and mechanical motion—are clearly forms of energy. Thus when we speak of stimulus energy, our meaning is straightforward. But what do we mean when we speak of stimulus energy when the stimulus is a chemical compound such as a sweet-tasting molecule? In these cases we are not referring to the bond energy of the molecule (the energy that would be released by burning or respiration). Instead, in the case of chemical stimuli, when the stimulus molecule binds with a protein during sensory reception, a change takes place in the protein’s conformation (molecular shape), and the energy involved in this process represents the stimulus energy.
715
Sensory receptor cells depend on specific receptor proteins and are ionotropic or metabotropic
A sensory receptor protein is a membrane protein in a sensory receptor cell that initially detects a stimulus and directly or indirectly produces a graded change, called a receptor potential, in the cell’s membrane potential. There are many types of sensory receptor proteins, and they respond to different kinds of stimuli. For example, some sensory receptor proteins respond to stretch. Others respond to particular chemicals. Still others respond to light. A receptor cell is specific in the sensory receptor protein it expresses, and this is one of the major reasons that receptor cells are highly specific in the stimuli to which they respond.
Receptor cells often have modified cell membranes with exceptionally large surface areas. The cell membrane, for example, may be greatly enlarged by the presence of microvilli, cilia, or membrane foldings. The enlarged cell membrane enables a receptor cell to have great numbers of receptor molecules, making the cell highly sensitive to stimuli:
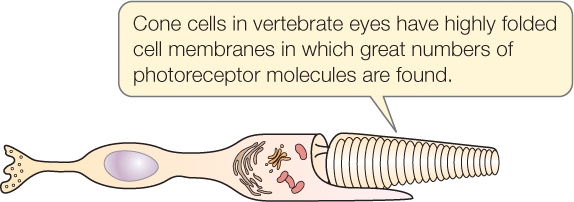
Sensory receptor cells may be ionotropic or metabotropic. The meanings of these terms in this context are similar to their meanings in the study of synapses (see Concept 34.3).
An ionotropic receptor cell typically has a receptor protein that is a stimulus-gated Na+ channel (FIGURE 34.12A). What happens during stimulus reception? First, the stimulus acts on the receptor protein. Second, the protein changes conformation so that it operates as an open Na+ channel, and Na+ diffuses in through the open channel, creating a receptor potential: a graded change in the cell membrane potential. Third, the graded change of membrane potential directly or indirectly results in action potentials.
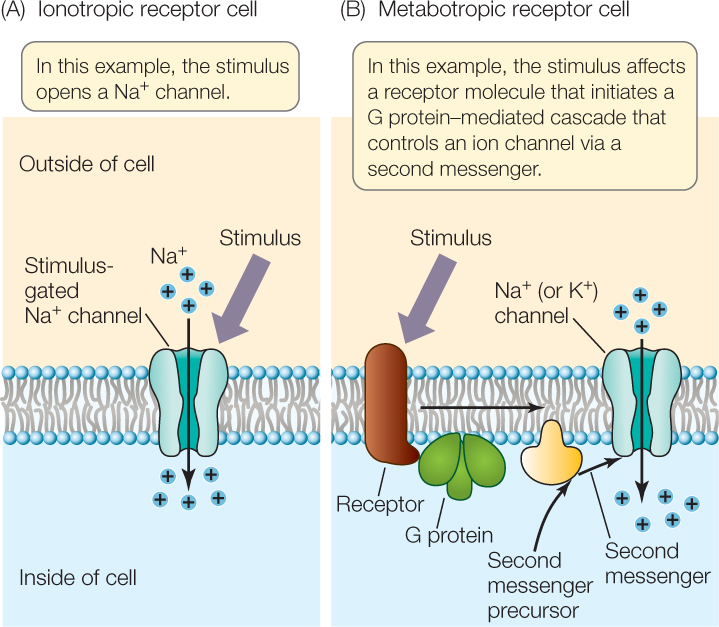
A metabotropic receptor cell typically has a receptor protein that activates a G protein when exposed to the stimulus (FIGURE 34.12B; see also Figure 5.14). In most cases, the receptor is a G protein–linked receptor. Activation of the G protein typically leads to a change in the concentration of a second messenger in the receptor cell. This change can directly or indirectly alter ion fluxes across the cell membrane, producing a receptor potential. The receptor cell itself may then generate action potentials, or it may secrete a neurotransmitter that affects another cell that produces action potentials.
Sensation depends on which neurons in the brain receive action potentials from sensory cells
All sensory systems convey information to the brain or other integrative parts of the nervous system in the form of action potentials. Accordingly, the brain receives action potentials from all types of sensory systems. How, then, do we perceive some of these action potentials as representing light, others as representing smells, and still others as representing sounds? How do we decode the action-potential codes?
Much of the answer lies in physical separation in the brain, between areas that receive axons from various sensory systems. In vertebrates, axons from the eyes travel to a specific brain region called the visual cortex. In turn, action potentials arriving in the visual cortex are interpreted as representing light or other visual sensations. Axons from the inner ear travel to another brain region called the auditory cortex, where arriving action potentials are interpreted as representing sounds. The intensity of the sensations (e.g., the brightness of a light or loudness of a sound) is coded by the frequencies of the action potentials.
We can see the importance of these principles on occasions when action potentials are generated by the wrong stimulus. People sometimes see flashes of light when they are poked in the eye. A strong poke can produce action potentials in axons from the retina that go to the visual cortex. Because the action potentials arrive in the visual cortex, the brain interprets them as representing light.
The parts of the brain that receive particular types of sensory information are determined during development, at least in part by interaction with the axons conveying the information. In a person blind from birth, for example, the visual cortex does not simply occupy space in the brain, unused, for life. Instead, much of the brain region is taken over to serve other sensory functions.
Sensation of stretch and smell exemplify ionotropic and metabotropic reception
Animals commonly have stretch receptor cells associated with their muscles. These are types of mechanoreceptors: cells that respond specifically to mechanical distortion of their cell membrane. When a muscle contracts, its stretching forces are applied to its stretch receptor cells, which provide the nervous system with information on the effectiveness of the contraction. Mechanoreceptors are typically ionotropic, as are thermoreceptors, which detect heat and cold.
716
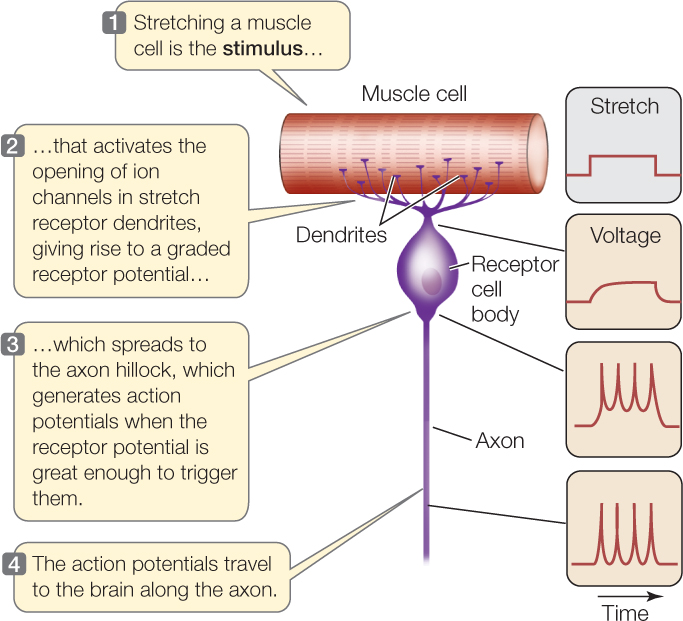
A stretch receptor cell of a crayfish provides a model of how an ionotropic receptor cell generates action potentials. The receptor cell is a specialized neuron. When the cell membranes of its dendrites are stretched, stretch-gated Na+ channels in the membranes open (see Figure 34.5B). Consequently, a graded, depolarizing receptor potential is produced and spreads to the axon hillock, and action potentials are generated there if the graded depolarization is above threshold (FIGURE 34.13). The rate at which action potentials are generated depends on the magnitude of the receptor potential, which depends on how much stretch the receptor cell detects.

Go to ANIMATED TUTORIAL 34.5 Mechanoreceptor Simulation
PoL2e.com/at34.5
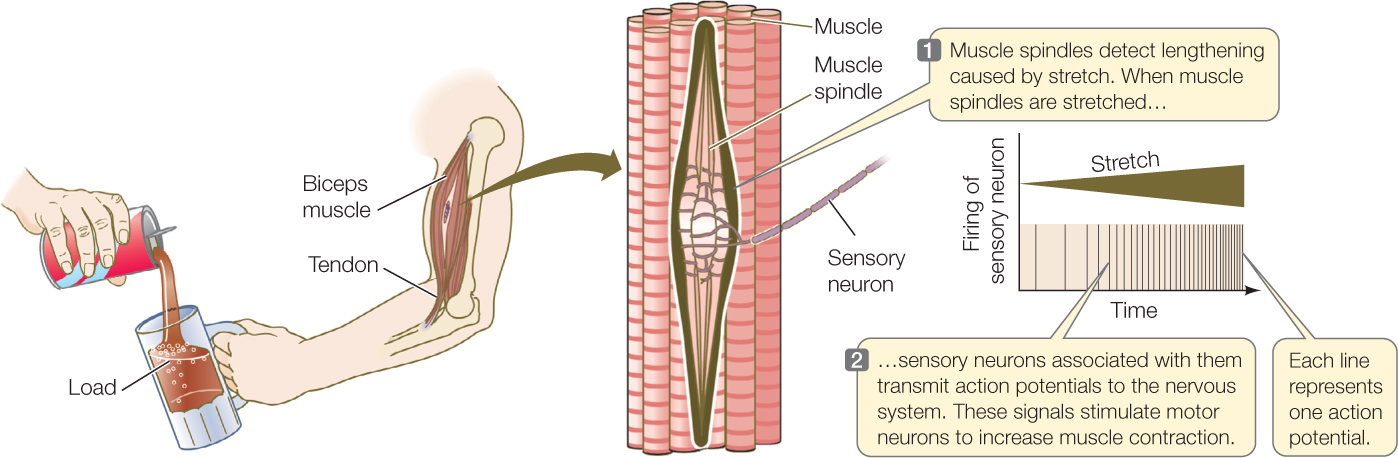
Stretch receptors associated with our biceps help us adjust the muscle’s strength of contraction to match the load the muscle must sustain (FIGURE 34.14). For example, suppose someone pours a drink into a glass you’re holding in your hand. Your biceps muscle tends to be stretched to a longer length as the weight of the glass increases. Receptors in the muscle detect this effect and encode it in the frequency of action potentials. Your nervous system then responds to the signals by stimulating stronger contraction as the glass fills, so that the glass does not pull your arm downward.
Now let’s look at an example of metabotropic reception, our sense of smell. Chemicals detected by smell are called odorants, and the sense of smell is called olfaction. The sensory cells for odorants, called olfactory receptor cells, are specialized neurons. They exemplify chemoreceptors: cells that respond to the presence or absence of specific chemicals. Mammalian olfactory receptor cells illustrate the workings of metabotropic sensory cells (see Figure 34.12B). They also illustrate in a spectacular way the importance of receptor protein diversity and specificity.
The olfactory receptor cells of a mammal are found in the olfactory epithelium, a part of the lining of the internal nasal cavity. One end of each receptor cell projects into the mucus that covers the surface of this epithelium. The other end of the cell is an axon that extends into a part of the brain called the olfactory bulb (see Figure 34.25). Odorants must dissolve in the liquid of the mucus to be detected.
The receptor protein of a mammalian olfactory receptor cell is a G protein–linked receptor found in the part of the cell that projects into the mucus. When an odorant molecule binds to the receptor, the receptor activates a G protein. The G protein in turn activates an enzyme that causes an increase of a second messenger in the cytoplasm. The second messenger opens ion channels in the cell membrane, causing a graded depolarization, which in turn causes action potentials to be generated in the cell. These propagate into the brain by way of the cell axon.
717
One of the amazing aspects of these cells is the number of different G protein–linked receptor proteins (GPLRs) they can express. Each cell expresses just one type of GPLR and can detect only the odorant molecules that bind to that GPLR. However, the genome codes for many options. About 1,000 different GPLRs are found in mice, for example. Collectively, the tens of thousands of olfactory receptor cells—each with one type of GPLR—can detect a very wide range of odorants. Each specific type of GPLR typically can bind to a range of odorants. However, different types of GPLRs have different sets of odorants they bind. The combination of olfactory cells that are stimulated by any particular odorant is unique to that odorant. Consequently, the brain can interpret the pattern of signals from the olfactory cells as pointing to a particular smell.
Some animals have olfactory receptor cells of extraordinary specificity. Because these cells effectively respond to only one ordinary odorant, they can be extremely sensitive to it. The feathery antennae of some male moths have huge numbers of odorant receptor cells that respond in a highly specific way to pheromone molecules emitted by the females of their species (see Concept 40.4). These moths can detect a female when her pheromone molecules represent only 0.000000000000001 percent of the molecules in the air:

Auditory systems use mechanoreceptors to sense sound pressure waves
Sounds consist of waves of compression of the air. An easy way to understand the nature of sound is to envision a loudspeaker. A close look reveals that the membrane of a loudspeaker moves back and forth. When the membrane moves toward you, it compresses the air, creating a region of relatively high pressure that moves toward you like a wave (at the speed of sound). When the speaker membrane moves away from you, it creates a region of relatively low pressure that also moves toward you between the regions of high pressure. You thus experience a sound pressure wave of alternating high and low pressure. The frequency of these alternations accounts for tone or pitch. We perceive high frequencies as high-pitch, treble tones and low frequencies as low-pitch, bass tones.
The hearing organs of many different types of animals are based on a single principle. The hearing organ contains a thin membrane that is free to move when a sound pressure wave strikes it—moving in when high pressure strikes and out when low pressure strikes. Specialized mechanoreceptors are linked to the membrane and detect the movements.
In an insect, the membrane is a simple structure in which a single area of membrane responds to all sound frequencies. A system of this sort is mainly useful for detecting sound presence or absence (and loudness), rather than discriminating one frequency from another.
The hearing system of mammals, by contrast, can separately detect many different frequencies. When we listen to someone playing the piano, our brain receives specific information on each frequency in the music, allowing us to perceive high notes as distinct from low notes.
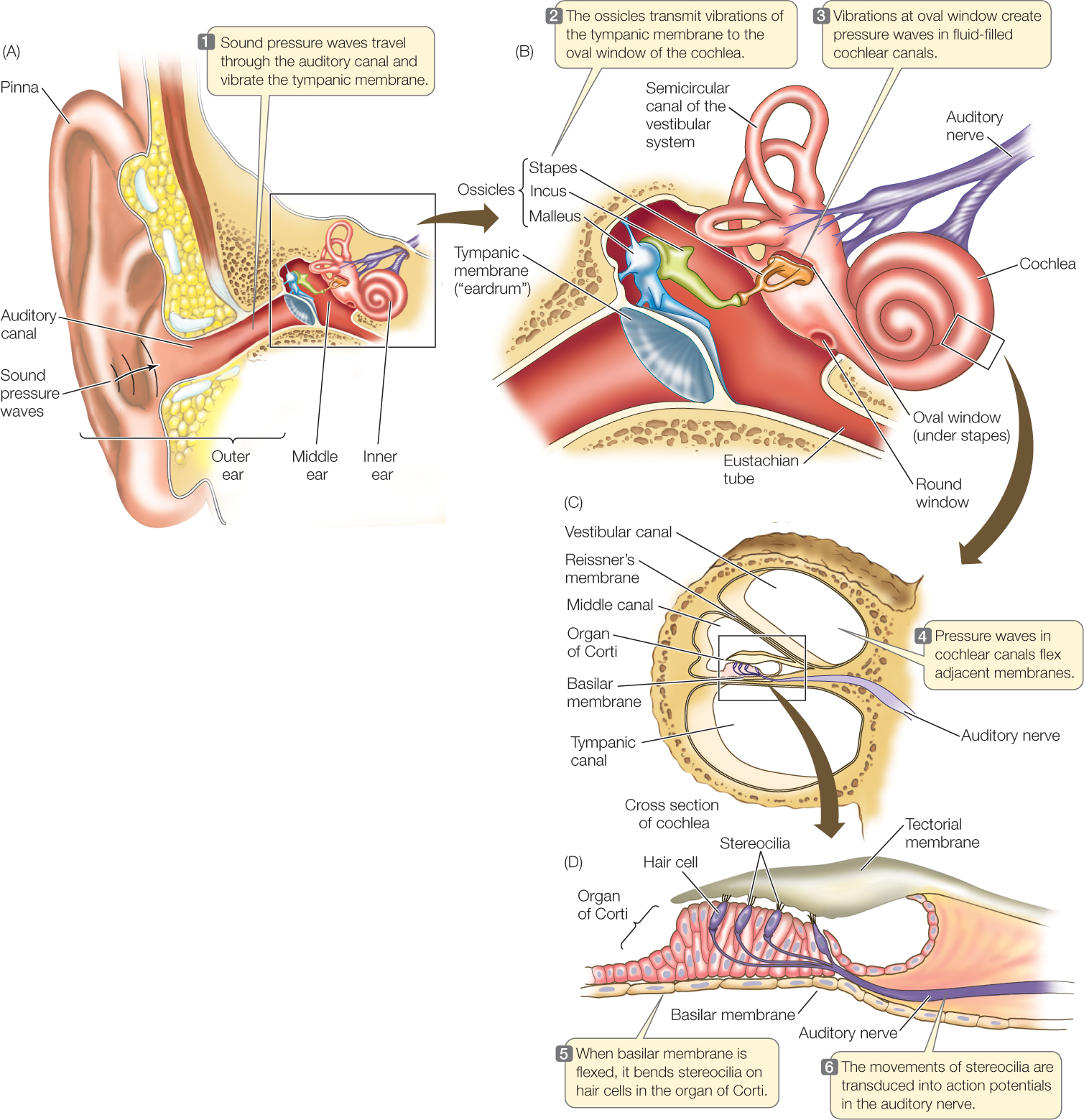
In the ear of a mammal, the membrane that initially responds to sound pressure waves is the tympanic membrane (ear drum). On the inside of the tympanic membrane is an air-filled cavity called the middle ear (FIGURE 34.15A). Because the tympanic membrane has air on both sides, it is free to vibrate when a sound pressure wave strikes it. Three interconnected, delicate bones—called ossicles and named the malleus, incus, and stapes—are found in the middle ear (FIGURE 34.15B). They act as a lever system that turns vibrations of the tympanic membrane into more forceful vibrations of another flexible membrane called the oval window.
Go to ACTIVITY 34.2 Structures of the Human Ear
PoL2e.com/ac34.2
The oval window is part of the inner ear. Specifically, it is part of the cochlea, a coiled, fluid-filled tube where sound energy is transduced into electric signals. A cross section of the cochlea reveals that it is composed of three parallel canals separated by two membranes. Of these the basilar membrane is of particular importance (FIGURE 34.15C).
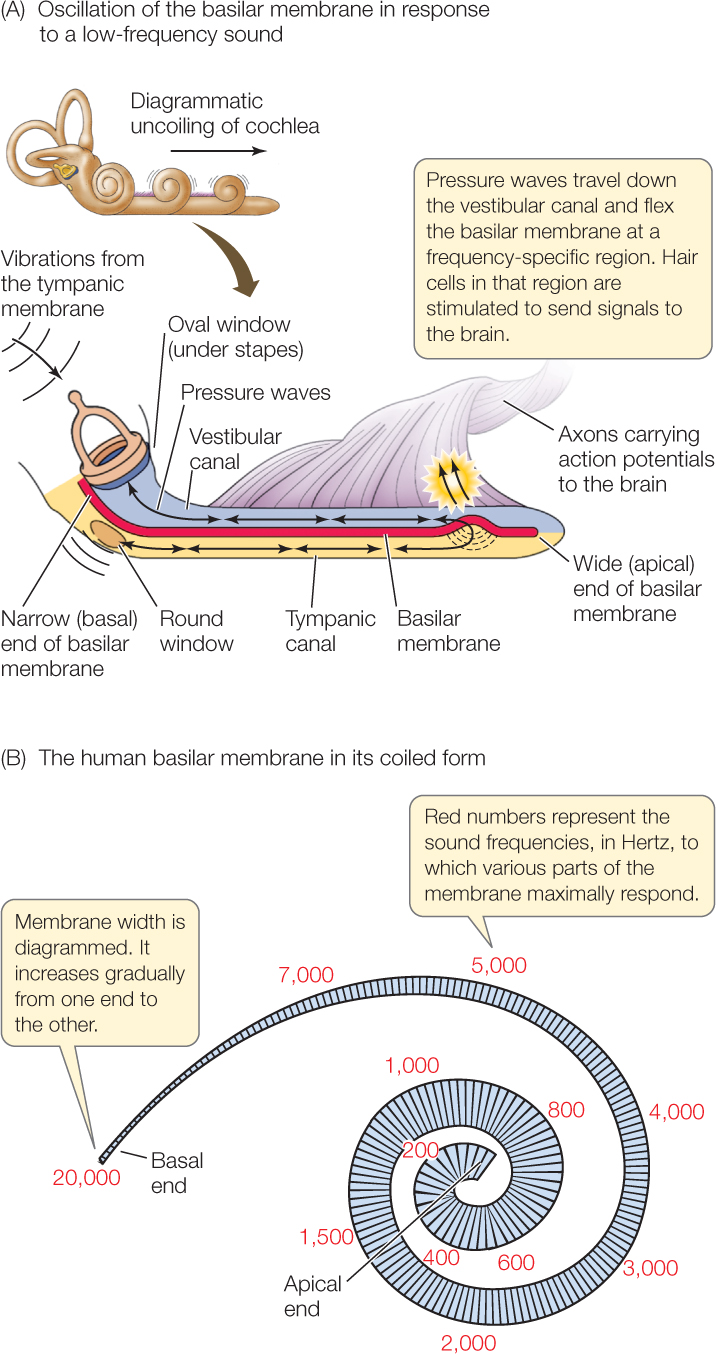
The basilar membrane gradually changes in width and stiffness from one end to the other. At the end nearest the oval window, it is only about 0.04 millimeters wide in the human ear and relatively stiff. It becomes progressively wider and more flexible until, at its far end, it is 0.5 millimeters wide and not as stiff.
When the sound pressure wave is transmitted into the cochlear fluid at the oval window, different frequencies in the sound pressure wave cause different, local regions of the basilar membrane to oscillate. Different regions move in response to different frequencies because of the gradations of membrane width and stiffness. Low-frequency vibrations (representing low pitches) have their greatest effect on the wide end of the membrane farthest from the oval window, setting that part into motion to a far greater degree than other parts (FIGURE 34.16A). Other frequencies affect other parts of the membrane (FIGURE 34.16B).

Go to ANIMATED TUTORIAL 34.6 Sound Transduction in the Human Ear
PoL2e.com/at34.6
How is information on these oscillations sent to the brain? Sitting on the basilar membrane is a structure, the organ of Corti, that houses distinctive mechanoreceptor cells called hair cells (FIGURE 34.15D). Each hair cell bears extensions called stereocilia (the “hairs,” which are actually microvilli, not cilia) that project into a semirigid membrane, the tectorial membrane. When a particular part of the basilar membrane oscillates, the stereocilia of the hair cells in that region are bent because they are effectively anchored at one end in the tectorial membrane but moving at the other end.
718
The hair cells transduce the bending motions of their stereocilia into electric signals. They do not produce action potentials. Instead, they synapse with neurons, and when they are stimulated by the bending of their stereocilia, they release a neurotransmitter that excites the neurons to produce action potentials, which travel to the brain. In this way, the frequency composition of a sound pressure wave is encoded by the particular hair cells that trigger action potentials as the wave exerts its effect in the inner ear (see Figure 34.16A).
The photoreceptors involved in vision detect light using rhodopsins
Both simple and complex animals have visual systems by which they sense and respond to light. The simplest visual systems give animals the ability to orient to the sun and sky. Complex visual systems provide animals with rapid and extremely detailed visual information about objects in their environments. Remarkably, the basic process of detecting light in visual systems has been conserved across the entire animal kingdom. Photoreceptors are sensory receptor cells that are sensitive to light. In all photoreceptors employed in vision, the sensory receptor protein is a member of a family of closely similar membrane pigments called rhodopsins. The rhodopsins act as G protein–linked receptors, and the photoreceptors are metabotropic receptor cells.
Detection of light depends on the ability of rhodopsins to absorb photons of light and undergo a change in molecular conformation (shape) as a consequence. A rhodopsin molecule consists of two parts: a protein, opsin (which alone is not photosensitive), and a nonprotein light-absorbing structure, 11-cis-retinal. The retinal part of each molecule is derived from vitamin A, explaining why sufficient vitamin A is essential for proper vision.
A rhodopsin molecule changes to a different isomer (arrangement of atoms in three-dimensional space) when it absorbs light. When the 11-cis-retinal in a molecule of rhodopsin absorbs light, it undergoes a twist around one of its bonds, so its atoms become reoriented in space to the all-trans-retinal isomer:
719
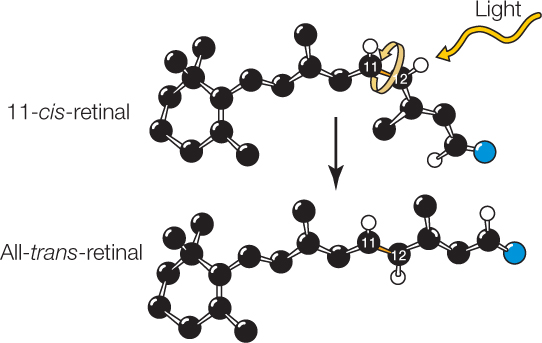
This change places strains on bonds in the opsin part of the molecule, changing the conformation of the opsin. The rhodopsin is thus activated. It in turn activates a G protein–mediated signal transduction cascade, in which a second messenger exerts effects on voltage-gated Na+ channels in the photoreceptor cell membrane.

Go to ANIMATED TUTORIAL 34.7 Photosensitivity
PoL2e.com/at34.7
Metabotropic control has a huge potential advantage in that it can greatly amplify signals. With large numbers of rhodopsin molecules and strong amplification, some photoreceptor cells undergo a measurable change in membrane potential in response to just a single photon of light!
LINK
Signal amplification by metabotropic control is described in Concept 5.6
The vertebrate retina is a developmental outgrowth of the brain and consists of specialized neurons
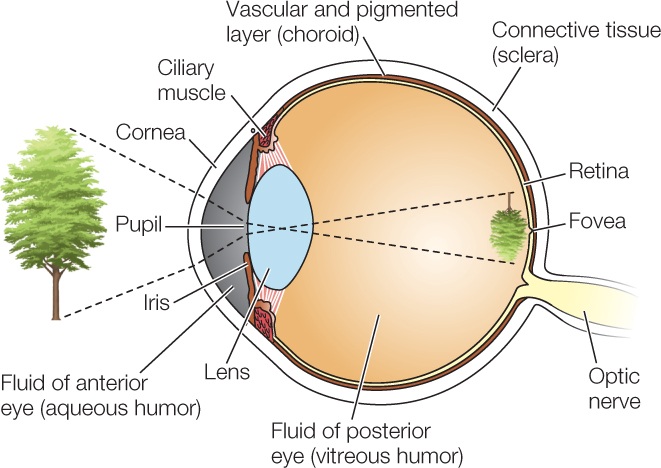
Vertebrates have image-forming eyes. Like a camera, an image-forming eye has a lens that focuses images on an internal surface that is sensitive to light. The eye is a fluid-filled structure bounded by a tough connective tissue layer, as exemplified by the human eye:
720
At the front of the eye, the connective tissue forms the transparent cornea. Just inside the cornea is the colored iris. Light enters the light-sensing part of the eye through the pupil, the central opening of the iris. The iris is equipped with small muscles that enlarge the diameter of the pupil in dim light and reduce it in bright light. Behind the iris is the lens, made of crystal-clear proteins called crystallins. In mammals, muscle cells pulling on the lens produce fine adjustments in its shape, focusing images on the photosensitive layer—the retina—at the back of the eye.
Go to ACTIVITY 34.3 Structure of the Human Eye
PoL2e.com/ac34.3
The retina is a developmental outgrowth of the brain and consists of several types of specialized neurons. Two types, the rod cells and cone cells, are the photoreceptor cells responsible for vision (FIGURE 34.17A). Both are specialized to have a large membrane area and large numbers of rhodopsin molecules. All rhodopsin molecules do not respond to the same wavelengths of light, even though their photoreceptive molecular subpart, the retinal, is the same. They differ in their opsins.
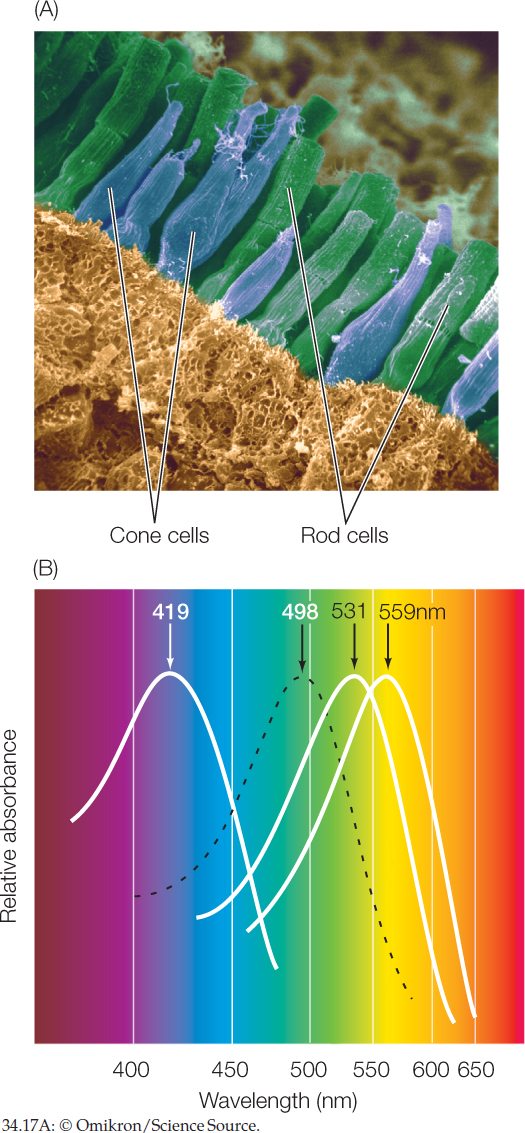
Rods outnumber cones in the human retina: 100 million rods versus 5 million cones. The rods are far more sensitive to light than the cones and are especially important for vision in dim light. Cones provide high-acuity color vision. Humans and some other primates have three types of cones, which are distinguished by having three different molecular forms of rhodopsin. These forms differ from one another in their sensitivities to various wavelengths of light, and all three differ from the single form of rhodopsin in rod cells (FIGURE 34.17B). As a group, the cone cells are able to detect and encode color because each wave-length of light has a unique collective effect on the three types.
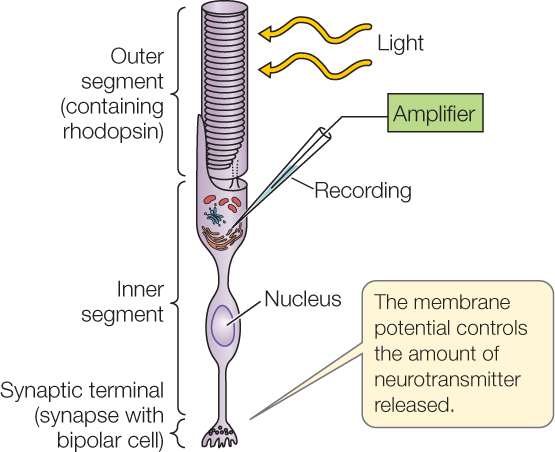
Rod and cone cells do not produce action potentials. They only produce graded membrane potentials. Compared with other sensory receptor cells, they are unusual in that they hyperpolarize in response to their stimulus. How does this work? When in darkness, they have open Na+ channels in their cell membranes. Na+ ions entering by way of these channels depolarize the cells to some extent. When the cells are stimulated by light, the second messenger produced under G protein control closes Na+ channels. This closing lowers the Na+ current flowing into the cell, permitting the charge on the inside of the cell membrane to become more negative relative to the charge on the outside. This effect is graded, depending on the light intensity (FIGURE 34.18). The rod and cone cells synapse with other neurons in the retina and relay signals to them by way of graded neurotransmitter release.
Investigation
HYPOTHESIS
When a rod cell absorbs photons (light energy), its membrane potential changes in proportion to the strength of the light stimulus.
METHOD
- Record membrane potentials from the inner segment of a rod cell.
- Stimulate the rod cells with light flashes of varying intensity and record the results.
RESULTS
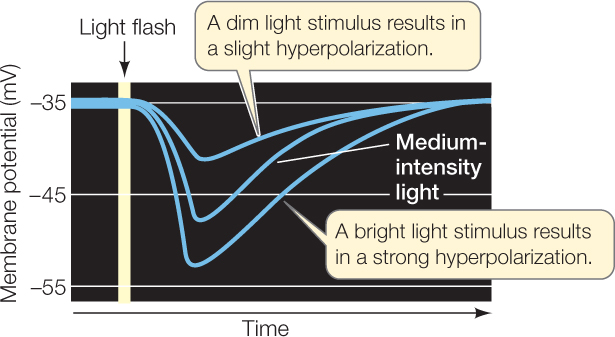
CONCLUSION
The membrane potential of rod cells is depolarized in the dark and hyperpolarizes (becomes more negative) in response to light.
Go to LaunchPad for discussion and relevant links for all INVESTIGATION figures.
a D. A. Baylor et al. 1979. Journal of Physiology 288: 589–611.
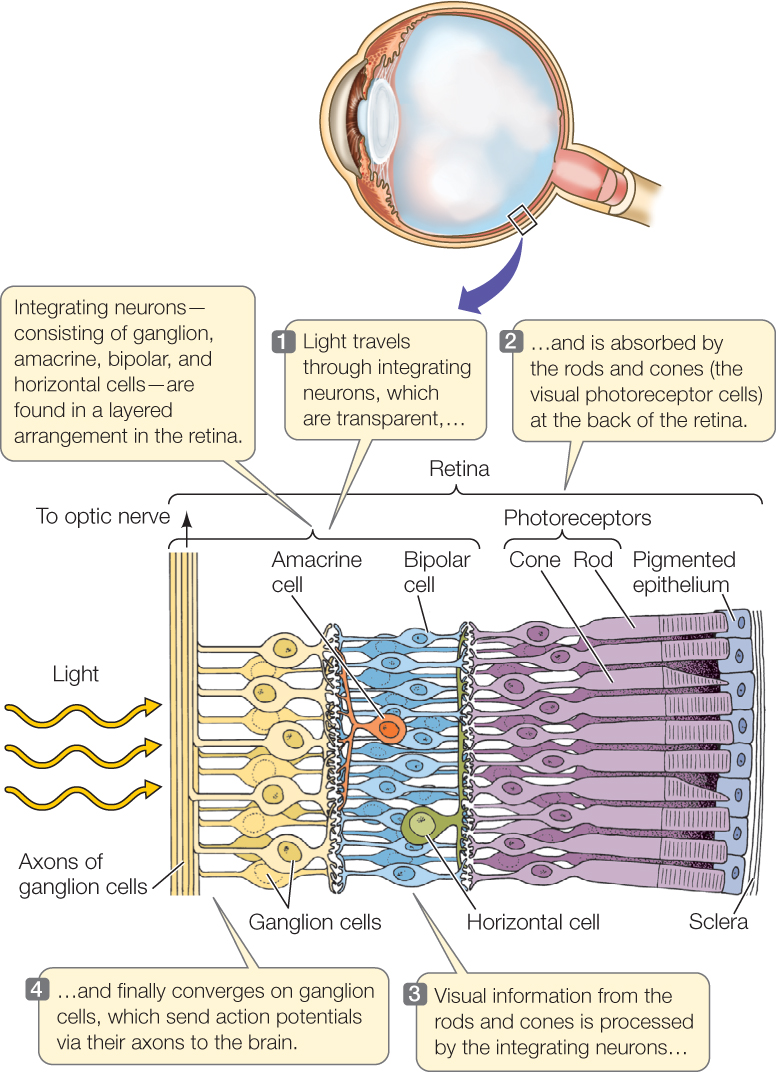
A great deal of signal processing occurs in the retina before visual signals are relayed to the brain by way of axons in the optic nerve. Besides the visual photoreceptor cells (rods and cones), four types of visual integrating neurons—called bipolar cells, horizontal cells, amacrine cells, and ganglion cells—are found in the retina in a layered arrangement (FIGURE 34.19; see also Figure 29.1). A striking feature of the arrangement of these cells is that light must pass through the integrating cells (which are transparent) to stimulate the rods and cones! This unexpected arrangement is a by-product of the embryonic developmental path by which the eyes form from the brain. The photoreceptor cells (rods and cones) synapse with bipolar cells, which in turn synapse with ganglion cells. In contrast to the cells in these straight-through connections from the back to the front of the retina, the horizontal cells and amacrine cells communicate laterally within the retina and in this way are able to extract information on the relative signal strengths in neighboring photoreceptor cells and neighboring bipolar cells. Horizontal cells help sharpen the perception of contrast between light and dark. Amacrine cells have several functions, including detecting motion. Ultimately, all of this information converges onto the ganglion cells, which are the only cells that produce action potentials. Axons of these cells converge to form the optic nerve and carry the action potentials to the visual cortex in the brain.
Go to ACTIVITY 34.4 Structure of the Human Retina
PoL2e.com/ac34.4

Go to MEDIA CLIP 34.2 Into the Eye
PoL2e.com/mc34.2
Each ganglion cell is said to have a defined, circular receptive field formed by the cells from which it receives signals. Some ganglion cells are excited by light falling on photoreceptor cells at the center of their receptive field and inhibited by light falling on photoreceptor cells in the periphery. Other ganglion cells are inhibited by light falling in the center of their receptive field and stimulated by light falling on the periphery. The result is that the ganglion cells can communicate information to the brain about visual patterns such as spots, edges, and areas of contrast.
721
The huge numbers of cells involved in vision say a lot about the amount of processing of visual images that takes place in the retina and brain. Each human retina sends a million axons to the brain. The visual cortex consists of hundreds of millions of neurons that receive and process the information from those million axons.
Some retinal ganglion cells are photoreceptive and interact with the circadian clock
One of the most revolutionary, recent discoveries about the mammalian eye is that 1–2 percent of the retinal ganglion cells are photoreceptive. Most ganglion cells (the other 98–99 percent) cannot directly detect light and function only to help integrate visual information that originates in the rods and cones.
The photoreceptive ganglion cells are not employed in vision. They have a relatively low sensitivity to light and do not produce images.
722
Rather than contributing to vision, these ganglion cells provide information simply on whether light is present and how bright it is. This information is used in two ways. It is relayed to the suprachiasmatic nuclei, where it is used to entrain the master circadian biological clock to the environmental rhythm of day and night (see Concept 29.6). The information is also employed in regulating the size of the pupils of the eyes, so the pupils are large in dim light and small in bright light.
The photoreceptive ganglion cells do not use rhodopsin. Instead, their sensory receptor protein is believed to be melanopsin.
Arthropods have compound eyes
Invertebrates have evolved a variety of types of eyes that differ from vertebrate eyes. Even the eyes of octopuses and squid, which look very similar to vertebrate eyes on the outside, have very different properties than vertebrate eyes.
The eyes of arthropods (insects, crayfish, crabs, and lobsters) are called compound eyes because each eye consists of many optical units, called ommatidia (singular ommatidium), each with its own lens. Light entering an ommatidium through its lens strikes photoreceptor (retinula) cells that contain rhodopsin and generate action potentials (FIGURE 34.20).
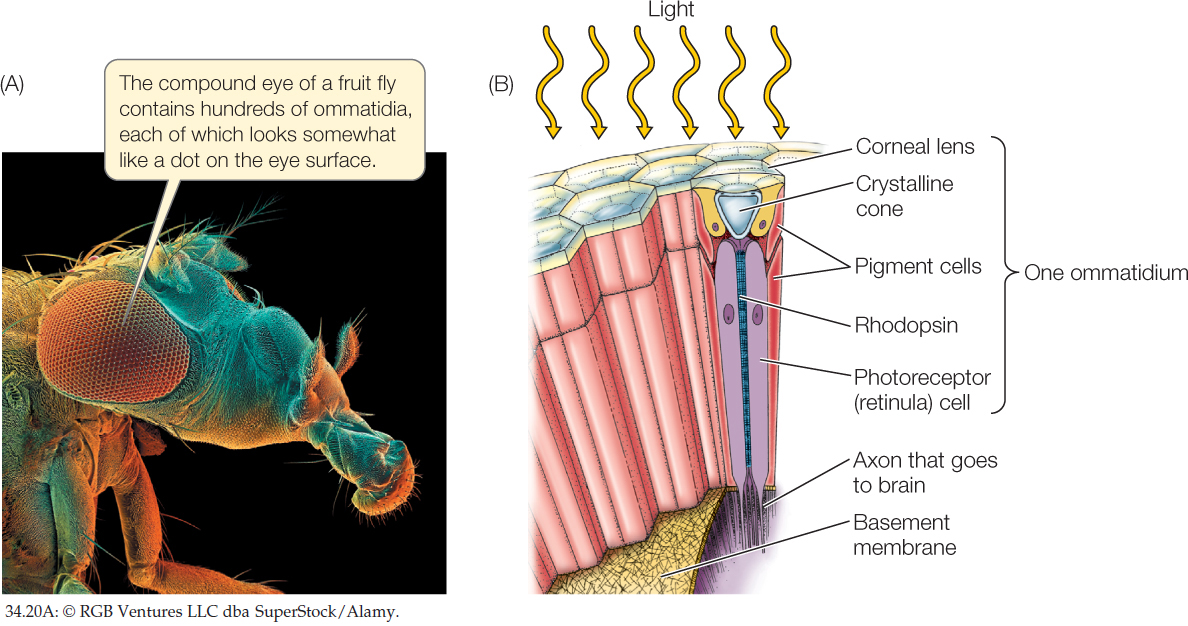
Each ommatidium of a compound eye is directed at a slightly different part of the visual world. The more ommatidia in the eye, the higher the resolution of the image compiled by the brain, rather like the pixel density in a computer monitor. The number of ommatidia in a compound eye varies from only a few in some ants to 800 in fruit flies and to 30,000 in some fast-flying predators such as dragonflies.
Animals have evolved a remarkable diversity of sensory abilities
Vision, hearing, olfaction, and touch are almost universal in animals. Animals use these sensory abilities to gather information about their external environment so they can move about effectively, avoid dangers, and exploit opportunities. Some animals, in addition, have evolved unusual sensory abilities that let them make use of additional information sources, such as electric fields or Earth’s magnetic field (see Figure 40.12). Moreover, some animals are able to use vision or hearing to gather information from parts of the electromagnetic spectrum or sound-frequency spectrum that are unfamiliar to us.
The philosopher Bertrand Russell titled one of his most famous books Our Knowledge of the External World. In this book he stressed that, as humans, we can only know about those aspects of our external environment that our human sensory systems detect. With this fact in mind, we realize that some animals that live around us perceive our external environment in very different ways than we do. They can see electromagnetic wavelengths to which we are blind or hear sound frequencies to which we are deaf.
The electromagnetic spectrum consists of electromagnetic energy at a broad range of wavelengths from 0 to over 100 micrometers (μm) (see Figure 6.16). Our eyes can see the wavelengths between 0.4 and 0.7 μm. We have names for the parts of the spectrum we cannot see: the wavelengths that are invisible to us even when our eyes are healthy. The wavelengths that are too long for our eyes to see (i.e., longer than 0.7 μm) are called infrared wavelengths. The wavelengths too short for our eyes to see (i.e., shorter than 0.4 μm) are called ultraviolet wavelengths.
Some snakes obtain vision-like images of their surroundings using infrared wavelengths. They are therefore able to see patterns of warmth and coolness in darkness—an ability that helps them find prey. The best-understood snakes with this ability are the pit vipers, such as rattlesnakes, which have specialized sense organs in pits near their eyes. The organ in each pit focuses infrared electromagnetic energy onto a membrane that can detect it, much like our eyes focus visible light onto our retina. Unlike the retina, the pit organ does not employ rhodopsins or similar molecules. Biologists still do not fully understand the sensory mechanism by which infrared electromagnetic energy is detected.
723

At the other end of the electromagnetic spectrum, many animals have eyes that can see ultraviolet wavelengths to which we are blind. Honey bees, for example, see flowers in different ways than we see them because they see in the ultraviolet. Many flowers that look uniform in color to our eyes have bold color patterns at the ultraviolet wavelengths bees see (see Figure 21.24). Many species of birds also see ultraviolet wavelengths. The eyes of these birds look much like ours. However, unlike the case in our eyes, their cornea and lens are transparent to ultraviolet wavelengths, permitting ultraviolet light to enter the eye, and the eye has specialized cone cells receptive to ultraviolet wavelengths.
The discovery of ultraviolet vision in birds led researchers to wonder, If the males and females of a species look identical to us, do they also look identical to the birds? Cedar waxwings (Bombycilla cedrorum), found almost everywhere in North America, are one species studied to answer this question. Male and female waxwings look the same to us. But we see only at 0.4–0.7 μm. When we use instruments to learn what the waxwings look like at shorter, ultraviolet wavelengths, we find that males and females look different. Thus the birds themselves can tell males and females apart by sight even though we cannot. This is equally true for many other species.
Some animals can sense electric fields—fields of voltage and current—enabling them to perceive their surroundings using information that’s entirely foreign to us. The weakly electric fish provide examples. These fish produce weak electric fields in their surroundings by use of modified muscles (see Concept 33.4). Electric currents in these self-produced environmental fields pass through the outer skin layers of the fish, where sensory receptor cells detect the currents. The fish detect distortions in these fields caused by objects in their external environment; by analyzing these distortions, the fish can perceive the objects even in complete darkness. Fish in the genus Gnathonemus use this sensory ability to locate and capture stream insects at night.

CHECKpoint CONCEPT 34.4
- As a mosquito lands on your arm, a cell in the mosquito’s leg responds to altered stretch within the leg. Another cell in its antennae detects the CO2 exhaled by you and other animals. What types of sensory receptor proteins do these cells probably contain?
- Referring to the structure of the vertebrate retina, explain how it is possible for visual signals to be processed extensively in the retina itself.
- A range of sound frequencies is changed to a range of locations in the vertebrate cochlea. How does this happen?
Now that we have discussed cellular processes from action potentials to receptor function, let’s examine how cells are organized to form the nervous systems of animals.