Concept 43.1: Interactions between Species May Increase, Decrease, or Have No Effect on Fitness
The interactions between leaf-cutter ants and their nest associates represent only a small fraction of the ants’ interactions with other species. As they harvest leaves, the ants interact with many plant species. The ants themselves are consumed by parasitic flies, beetles, and pathogenic fungi, and the ant corpses that are hauled to the colony’s refuse dump are consumed by many species of microbes. The leaf-cutter example illustrates one of life’s certainties: at some point between birth and death, every organism will encounter and interact with individuals of other species. Interspecific (Latin, “between species”) interactions affect each individual’s life history, and thereby its success in surviving and reproducing, which in turn determines the individual’s contribution to total population growth rate (see Concept 42.3; keep in mind that males contribute to the production of new individuals by inseminating females). This contribution to population growth rate is a measure of that individual’s fitness (see Concept 15.4). By affecting survival and reproduction, interspecific interactions influence the dynamics and densities of populations, alter the distributions of species, and lead to evolutionary change in one or both of the interacting species.
Interspecific interactions are classified by their effects on fitness
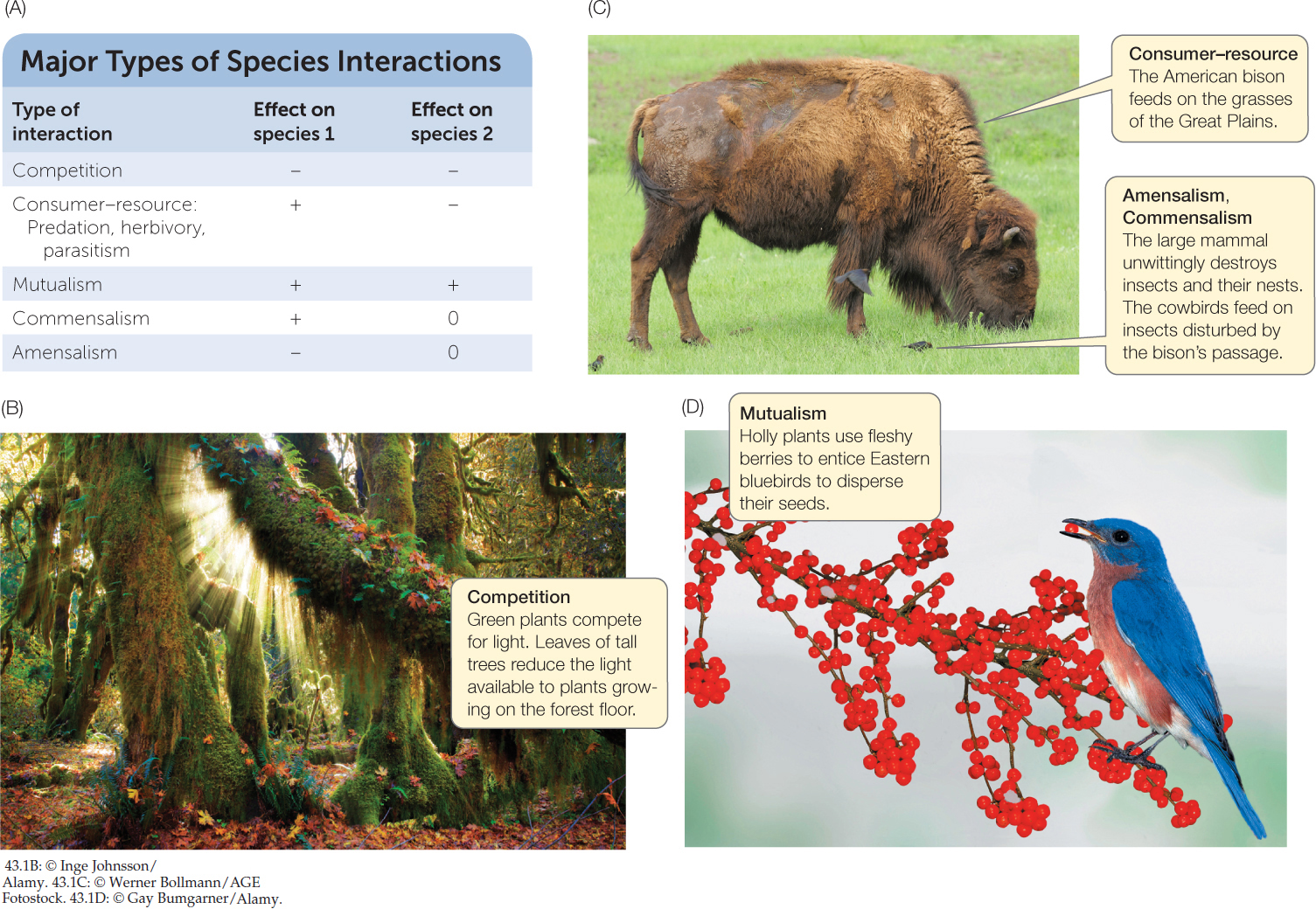
The details of interspecific interactions are bewilderingly diverse, but we can cut through all those details by asking, “Does the interaction help or harm individuals of the participating species?” Or more specifically, “Does the interaction increase, decrease, or have no effect on survival and reproduction, and thus on fitness?” If we keep these questions in mind, we will see that there are only five broad categories of interspecific interactions (FIGURE 43.1A): competition (−/−), consumer–resource (+/−), mutualism (+/+), commensalism (+/0), and amensalism (−/0).
Go to ACTIVITY 43.1 Ecological Interactions
PoL2e.com/ac43.1
884
Interspecific competition refers to −/− interactions in which members of different species decrease one another’s fitness because they require some of the same resources. Recall from Concept 42.4 that when an organism uses a resource, that resource becomes unavailable to other organisms, potentially reducing their survival and reproduction. We say “potentially” because not all resources are in short enough supply to limit these components of fitness. However, at any one time there is generally some limiting resource that is in the shortest supply relative to demand. Species that share limiting resources are likely to compete.
Competition may occur among organisms that consume the same food, whether they are African lions and spotted hyenas that attack wildebeest, or aphids and grasshoppers that eat the same plant. Even the interaction between the mold Escovopsis and leaf-cutter ants can be considered competitive, because both derive energy and nutrients from the fungus that the ants cultivate. But the limiting resource need not be food. Plants may be limited by availability of sunlight (FIGURE 43.1B) or inorganic nutrients in the soil. Some organisms are limited by water or space in which to grow (think of barnacles encrusting a rocky shore; see Figure 43.4) or in which to build a nest. Plants may even compete for the attention of animal partners that pollinate flowers or disperse seeds and thereby contribute to the plants’ reproduction.
Consumer–resource interactions are those in which organisms gain their nutrition by consuming other living organisms or are themselves consumed. It’s obvious why these are +/− interactions: the consumer benefits while the consumed organism—the resource—loses. Consumer–resource interactions include predation, in which an individual of one species (the predator) kills and consumes individuals of another species (the prey); herbivory, in which an herbivore consumes part or all of a plant (FIGURE 43.1C); and parasitism, in which a parasite consumes part of a host individual but usually does not kill it. These categories blur together to some degree. For example, herbivorous caterpillars that consume some of a plant’s leaves (see Figures 28.4 and 28.5) are functionally similar to the parasitic black-legged ticks (see Figure 42.3) that consume some of a rodent’s blood. And some parasites are functionally similar to predators because they consume so much of the host’s body, or otherwise damage it so severely (e.g., some pathogenetic organisms; see pp. 393–394 and 454–455), that the host dies.
A mutualism is an interaction that benefits both species (+/+). The interaction between leaf-cutter ants and the fungus they cultivate is mutualistic: the ants feed, cultivate, and disperse the fungus; the fungus, in turn, converts inedible leaf fragments into special fungal structures that the ants can eat. The interactions between plants and pollinating or seed-dispersing animals (FIGURE 43.1D), or between humans and some of their gut bacteria (see Concept 41.1), are similarly beneficial to both partners. Mutualisms take many forms and involve many kinds of organisms. They also vary in how essential the interaction is to the partners.
LINK
We have seen several examples of mutualisms in this book, including interactions between mycorrhizal fungi and plants (see Concepts 22.2 and 25.2); between fungi, algae, and cyanobacteria in lichens (see Concept 22.2); and between corals and dinoflagellates (see Concept 20.4).
Competition, consumer–resource interactions, and mutualism all affect the fitness of both participants. The other two defined types of interactions affect only one of the participants.
In commensalism, one participant benefits while the other is unaffected (+/0). Some examples involve species whose feeding behavior makes food more accessible for another. For example, the brown-headed cowbird (Molothrus ater) owes its common name to its habit of following herds of grazing cattle, foraging on insects flushed from the vegetation by their hooves and teeth. (M. ater has also been called the buffalo bird because it followed the bison herds that were once abundant across North America; see Figure 43.1C). The ungulate–cowbird interaction is commensal because the birds have no effect on ungulate fitness. In other cases, the feeding of one species benefits a second species by converting food into a form it can use. Cattle, for example, convert plants into dung, which dung beetles (see Figure 44.4) can use, but the dung beetles have no effect on the cattle. Dung beetles, in turn, are on the giving end of another commensalism: dung-dwelling organisms that cannot fly, such as mites, nematodes, and even fungi, attach themselves to the bodies of the beetles, which not only can fly but are very good at locating fresh dung. The hitchhikers have no apparent effect on dung beetle survival or reproduction.
Amensalism refers to interactions in which one participant is harmed while the other is unaffected (−/0). Elephants moving through a forest or bison grazing the plains crush insects and plants with each step (see Figure 43.1C), but the large mammals are unaffected by this carnage. Amensal interactions tend to be more accidental in nature than the other interactions, as these examples suggest.
The effects of many interactions are contingent on the environment
Ecologists find it useful to distinguish the five categories of interspecific interactions just described, but real interactions can be hard to categorize. Many interactions have both beneficial and harmful aspects, and the net effect of one species on another can be positive or negative depending on environmental conditions.
Sea anemones, for example, sting and consume small animals, including fish. But a select few fish species (mostly anemonefishes; genus Amphiprion) are protected by a special mucus coat and thus can live among the anemones’ stinging tentacles (FIGURE 43.2). Although the benefit of this association to the anemonefishes is clear—they escape their own predators by hiding behind the anemones’ nematocysts and can scavenge food caught by the anemones—more research is needed to clarify the consequences for the anemones. Are they nourished by the fishes’ nitrogen-rich feces? Do they suffer because the fish steal some of their prey? The net effect of the interaction for anemones may change from beneficial when food is scarce to detrimental when food is abundant.

885
APPLY THE CONCEPT: Interactions between species may increase, decrease, or have no effect on fitness
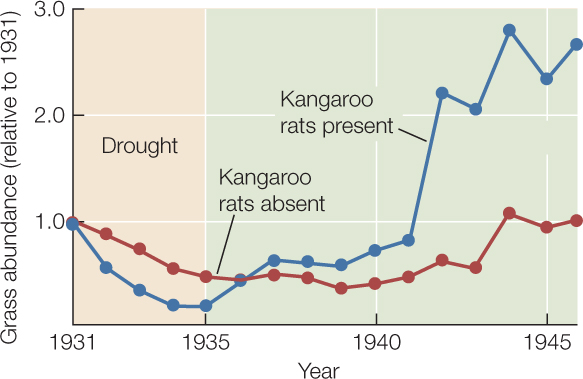
Many animals that face uncertain food availability have evolved a tendency to collect and store more food than they can eat right away. Desert seed-eating rodents such as Merriam’s kangaroo rat (Dipodomys merriami; see Figure 42.4B), for example, will harvest as many seeds as you put in front of them and bury the seeds in shallow deposits scattered around their territories. In some years, the plants in the desert environment produce few seeds, and the kangaroo rats eat all the seeds they have stored. In other years, the kangaroo rats store more seeds than they are able to eat, and the uneaten seeds germinate—they have been “planted.” In those years, the plant species whose seeds kangaroo rats favor increase in abundance.
Researchers in southern Arizona monitored the effect of kangaroo rats on the large-seeded native bunchgrasses that the animals prefer. They fenced several plots of land with rodentproof fences and removed kangaroo rats from half the plots. Over the next 15 years they compared the area covered by the grasses (a measure of their abundance) in plots with and without kangaroo rats.a
From 1931 to 1935, southern Arizona experienced a severe drought, and grass populations declined. Rains returned in 1935, and the year 1941 saw particularly high rainfall (for the desert). Examine the graph showing differences in grass abundance (relative to 1931 values) between the two types of plots and answer the following questions.
- During the drought, did kangaroo rats have a positive, negative, or neutral effect on the grasses? Explain your reasoning.
- Did the effect of kangaroo rats on grasses change after rains returned? Explain your reasoning.
- What aspects of kangaroo rat behavior are detrimental to grasses? What aspects might help grasses?
- Imagine a hypothetical kangaroo rat species that finds and eats 50% of the seeds in its environment without storing any. Redraw the blue line in the figure for this hypothetical animal.
aH. G. Reynolds. 1950. Ecology 31: 456–463.
CHECKpoint CONCEPT 43.1
- What is the criterion for classifying interspecific interactions?
- What type of interspecific interaction occurs between humans and their crop plants?
- Describe an experiment that would allow you to determine whether the effect of anemonefishes on sea anemones is positive, negative, or neutral.
Interspecific interactions affect population dynamics because they influence the contributions of individuals to population growth rate. How exactly are population dynamics affected?