Chapter 4. CASE 4: MALARIA: CO-EVOLUTION OF HUMANS AND A PARASITE
CASE 4: MALARIA: CO-EVOLUTION OF HUMANS AND A PARASITE
C4-2
Most people would say the world has more than enough mosquitoes, but in 2010 scientists at the University of Arizona conjured up a new variety. The high-tech bloodsuckers were genetically engineered to resist Plasmodium, the single-celled eukaryote that causes malaria.
Normally, the parasite grows in the mosquito’s gut and is spread to humans by the insect’s bite. By altering a single gene in the mosquito’s genome, the researchers had made the insects immune to the malaria parasite. The accomplishment is a noteworthy advance, the latest in a long line of efforts to stop Plasmodium in its tracks.
Malaria is one of the most devastating diseases on the planet. The World Health Organization estimates that 500 million people contract malaria annually, primarily in tropical regions. The disease is thought to claim about a million lives each year. Of those deaths, 85% to 90% occur in sub-Saharan Africa, mostly among children under 5.
Five species of Plasmodium can cause malaria in humans. One of them, P. falciparum, is particularly dangerous, accounting for the vast majority of malaria fatalities. Over thousands of years, this parasite and its human host have played a deadly game of tug-of-war. As humans have tried a succession of weapons to defeat P. falciparum, the parasite has evolved, thwarting their efforts. And in turn, the tiny organism has helped to shape human evolution.
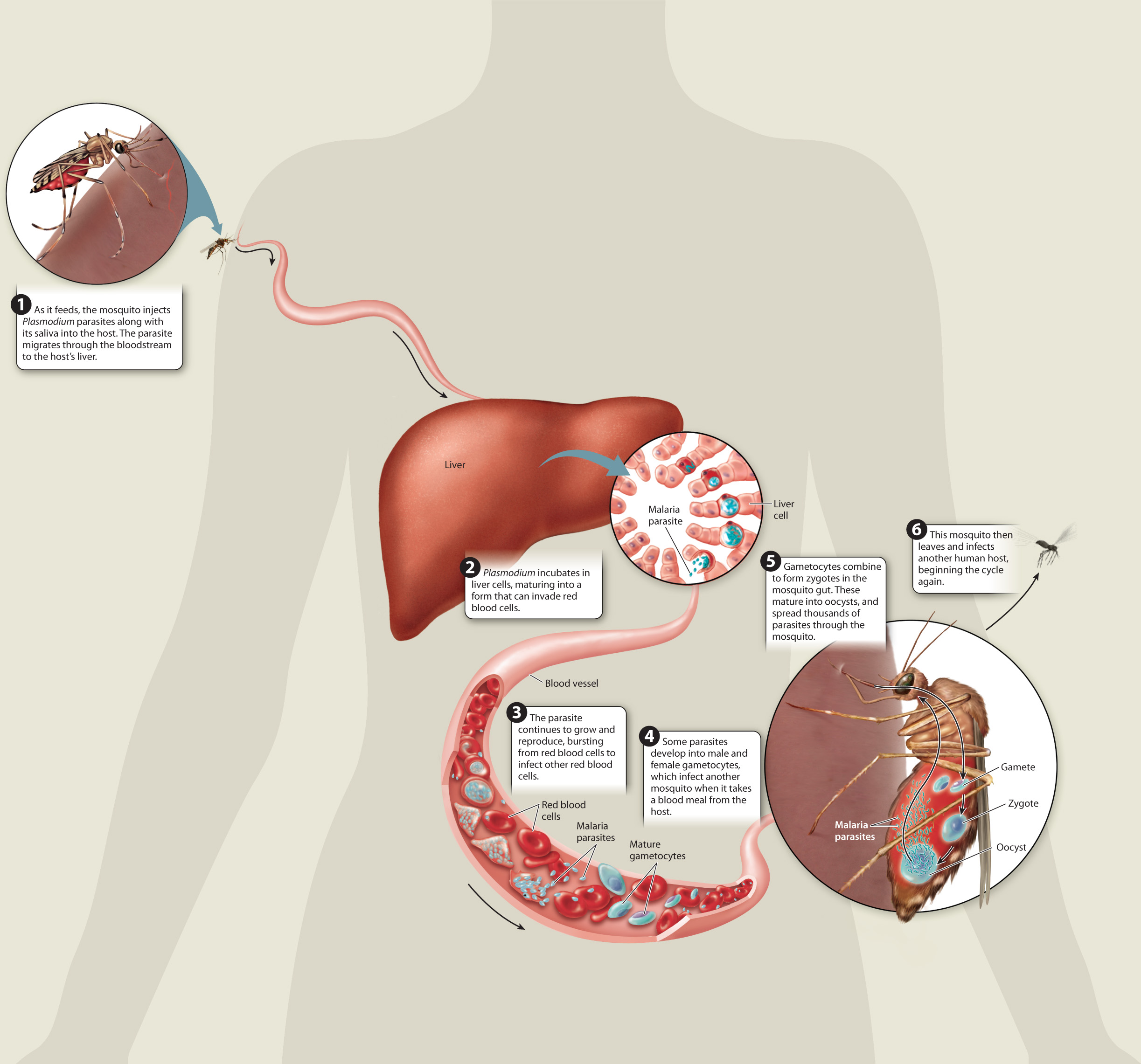
Plasmodium is a wily and complicated parasite, requiring both humans and mosquitoes to complete its life cycle. The part of the cycle in humans begins with a single bite from an infected mosquito. As the insect draws blood, it releases Plasmodium-laden saliva into the bloodstream. Once inside their human host, the Plasmodium parasites invade the liver cells. There they undergo cell division for several days, their numbers increasing. Eventually, they infect red blood cells, where they continue to grow and multiply. The mature parasites burst from the red blood cells at regular intervals, triggering malaria’s telltale cycle of fever and chills.
Some of the freed Plasmodium parasites go on to infect new red blood cells; others divide to form gametocytes that travel through the victim’s blood vessels. When another mosquito bites the infected individual, it takes up the gametocytes with its blood meal. Inside the insect, the parasite completes its life cycle. The gametocytes fuse to form zygotes. Those zygotes bore into the mosquito’s stomach, where they form oocysts that give rise to a new generation of parasites. When the infected mosquito sets out to feed, the cycle begins again.
The battle between malaria and humankind has raged through the ages. Scientists have recovered Plasmodium DNA from the bodies of 3500-year-old Egyptian mummies —evidence that those ancient humans were infected with the malaria parasite. The close connection between humans, mosquitoes, and the malaria parasite almost certainly extends back much further.
As humans have tried a succession of weapons to defeat P. falciparum, the parasite has evolved, thwarting their efforts.
In fact, people who hail from regions where malaria is endemic are more likely than others to have certain genetic signatures that offer some degree of protection from the parasite. That indicates that Plasmodium has been exerting evolutionary pressure on humankind for quite some time.
C4-3
Meanwhile, we’ve done our best to fight back. For centuries, humans fought the infection with quinine, a chemical found in the bark of the South American cinchona tree. In the 1940s, scientists developed a more sophisticated drug based on the cinchona compound. That drug, chloroquine, was effective, inexpensive, easy to administer, and caused few side effects. As a result, it was widely used, and in the late 1950s, P. falciparum began showing signs of resistance to chloroquine. Within 20 years, resistance had spread to Africa, and today most strains of P. falciparum have evolved resistance to the once-potent medication.
Just as bacteria develop resistance to antibiotics, Plasmodium evolves resistance to the antiparasitic drugs designed to fight it. In poor, rural areas where malaria is prevalent, people often can’t follow the recommended protocols for antimalarial treatment. Sick individuals may be able to afford only a few pills rather than the full recommended dose. The strength and quality of those pills may be questionable, and the drugs are often taken without oversight from a medical professional.
C4-4
Unfortunately, inadequate use of the drugs fuels resistance. When the pills are altered or the course of treatment is abbreviated, not all Plasmodium parasites are wiped out. Those that survive in the presence of the drug are likely to evolve resistance to the drug. Because the resistant parasites have a survival advantage, the genes for drug resistance spread quickly through the population.
Since chloroquine resistance emerged, pharmaceutical researchers have developed a variety of new medications to prevent or treat malaria. Most, however, are far too expensive for people in the poverty-stricken regions where malaria is rampant. And just as was the case with chloroquine, almost as quickly as new drugs are developed, Plasmodium begins evolving resistance.
One of the more recent weapons added to the drug arsenal is artemisinin, a compound derived from the Chinese wormwood tree. It has turned out to be an effective and relatively inexpensive way to treat malaria infections. However, pockets of artemisinin resistance have already been uncovered in Southeast Asia. Public health workers now recommend that artemisinin be given in combination with other drugs. Treating infected patients with multiple drugs is more likely to wipe out Plasmodium in their bodies, reducing the chances that more drug-resistant strains will emerge.
Given the challenges of developing practical drugs to prevent or treat malaria, some scientists have turned their attention to other approaches. One goal is to produce a malaria vaccine. The parasite’s complex life cycle makes that a complicated endeavor, though. Several vaccines are now in various stages of testing, and some show promise. But researchers expect it will be years before a safe, effective vaccine for malaria could be available.
Other researchers are focusing their efforts on the mosquitoes that carry the parasite, rather than on Plasmodium itself. The genetically engineered insects created by the team in Arizona are a promising step in that direction.
The Arizona researchers set out to alter a cellular signaling gene that plays a role in the mosquito’s life cycle. Mosquitoes normally live 2 to 3 weeks, and Plasmodium takes about 2 weeks to mature in the mosquito’s gut. The researchers hoped to create mosquitoes that would die prematurely, before the parasite is mature. The genetic modification worked as planned. The engineered mosquitoes’ life-spans were shortened by 18% to 20%. The genetic tweak also had a surprising side effect. The altered gene completely blocked the development of Plasmodium in the mosquitoes’ guts. The engineered mosquitoes are incapable of spreading malaria to humans, regardless of how long they live.
While the finding was a laboratory success, it will be much harder to translate the results to the real world. To create malaria-free mosquitoes in the wild, scientists would have to release the genetically modified mosquitoes and hope that their altered gene spreads through the wild mosquito population. But that gene would be passed on only if it gave the insects a distinct evolutionary advantage. The engineered mosquitoes may be malaria free, but so far they’re no fitter than their wild counterparts.
It’s clear that slashing malaria rates will not be an easy task. Despite decades of research, insecticide-treated bed nets are still the best method for preventing the disease. For millennia, the malaria parasite has managed to withstand our efforts to squelch it, yet science continues to push the boundaries. Who will emerge the victor? Stay tuned.
Questions Arising from Case 4
Answers to Case 4 questions can be found in Chapters 21-24.
- What genetic differences have made some individuals more and some less susceptible to malaria? See page 21-10.
- How did malaria come to infect humans? See page 22-11.
- What human genes are under selection for resistance to malaria? See page 24-15.