Chapter 5. CASE 5: THE HUMAN MICROBIOME: DIVERSITY WITHIN
CASE 5: THE HUMAN MICROBIOME: DIVERSITY WITHIN
C5-1
Clostridium difficile doesn’t grab many headlines. Perhaps it should. This bacterium is thought to be the most common cause of hospital-acquired infections in the United States. It sickens about 165,000 U.S. hospital patients each year, causing fevers, chills, diarrhea, and severe intestinal pain—and adds more than $1 billion annually to the nation’s health care costs. Altogether, the infection kills about 28,000 people every year, according to the Centers for Disease Control and Prevention.
Now a promising (if stomach-turning) new treatment is gaining traction: fecal transplants. The vast majority of C. difficile cases occur after patients have taken antibiotics for other infections. The drugs kill the beneficial bacteria normally found in a healthy gut, allowing C. difficile to proliferate. Now some pioneering researchers have begun to replace the missing microbes by transplanting fecal material from healthy donors into patients sick with C. difficile. Some doctors have reported that the procedure alleviates symptoms in more than 80% of patients.
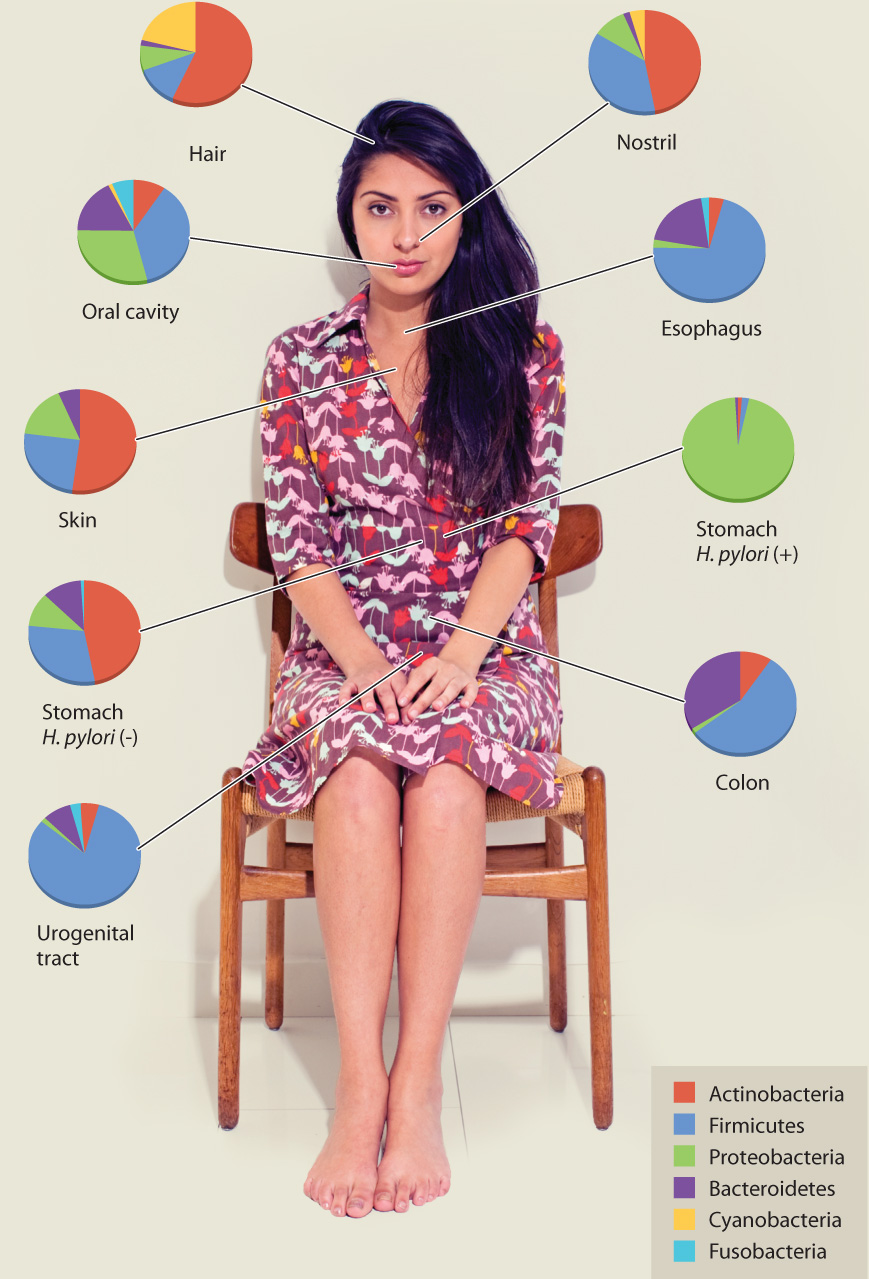
While transplanting fecal bacteria may not yet be mainstream medicine, there is no question that our gut, as well as our skin, mouth, nose, and urogenital tract, is home to a diversity of bacterial species. No one has yet identified the many microbes living on and within our bodies, but estimates suggest that each of us contains 10 times as many bacterial cells as human cells. The relationship between “us and them” is a type of symbiosis, in which both humans and bacteria benefit from the close association. Indeed, the microbiome, as our assemblage of bacterial houseguests is known, affects human health in many important ways.
Some of the most mysterious, and most intriguing, members of our microbiome live in our gut. Bacteria living in our intestines help us break down food and synthesize vitamins. On the other hand, abnormal communities of microbes in the gut have been linked to Crohn’s disease, colon cancer, and nonalcoholic fatty liver disease.
No one has yet identified the many microbes living on and within our bodies, but estimates suggest that each of us contains 10 times as many bacterial cells as human cells.
Your own personal gut flora is a unique collection of species—even identical twins can have surprisingly different microbiomes. This variability has made it difficult for scientists to decipher exactly what role individual species play in health and in disease. The sheer number of bacteria in your gut only compounds the problem. One international team of scientists recently reported that each human gut contains at least 160 different bacterial species.
Despite these challenges, researchers have made strides toward understanding the unique bacterial communities within our intestinal tract. In 2011, scientists compared all the DNA sequences from gut microbes of people living in Europe, America, and Japan. They found that each individual fell into one of three enterotypes, or “gut types.” Most people had a bacterial mix dominated by organisms from the genus Ruminococcus. But others had gut types dominated by the genus Bacteroides or the genus Prevotella. The three different gut compositions appear to produce different vitamins and may even make a person more, or less, susceptible to certain diseases.
Surprisingly, the researchers reported that enterotype was unrelated to gender, body mass index, or nationality. So what determines your gut type? A follow-up study suggested that enterotypes were associated with long-term dietary patterns. People who eat a diet high in animal protein and saturated fats are more likely to contain a gut community dominated by Bacteroides. People who stick to a high-carbohydrate diet rich in plant-based nutrition are more likely to host an abundance of Prevotella.
C5-2
The researchers don’t know yet what a person’s enterotype means for his or her health. But scientists are eager to explore the link between microbiome and physical well-being. Meanwhile, various researchers are exploring the microbiome of other organisms—specifically, cows.
As it happens, there are good reasons to peer into a cow’s gut. The cow’s rumen (one of the four stomach chambers) contains microbes that break down the cellulose in plant material. Without them, cattle wouldn’t be able to extract adequate nutrition from the grass they graze. Those microbes are of particular interest to scientists at the Department of Energy (DOE), who are interested in converting material containing cellulose, such as switchgrass, into biofuels.
As researchers look for more sustainable replacements for fossil fuels, many have pinned their hopes on biofuels. But the enzymes currently used to break down cellulose aren’t efficient or cost effective enough to produce biofuels on an industrial scale. That’s where the cows come in. DOE scientists recently analyzed the microbial genes present in the cow rumen. They discovered nearly 28,000 genes for proteins that metabolize carbohydrates, and at least 50 new proteins that may help break down cellulose in ways that will produce biofuels more efficiently and more cheaply.
C5-3
Mammals such as cows and humans are hardly the only organisms that have symbiotic relationships with bacteria. All living animals have their own microbiomes. Even tiny single-celled eukaryotes harbor still-tinier bacterial guests.
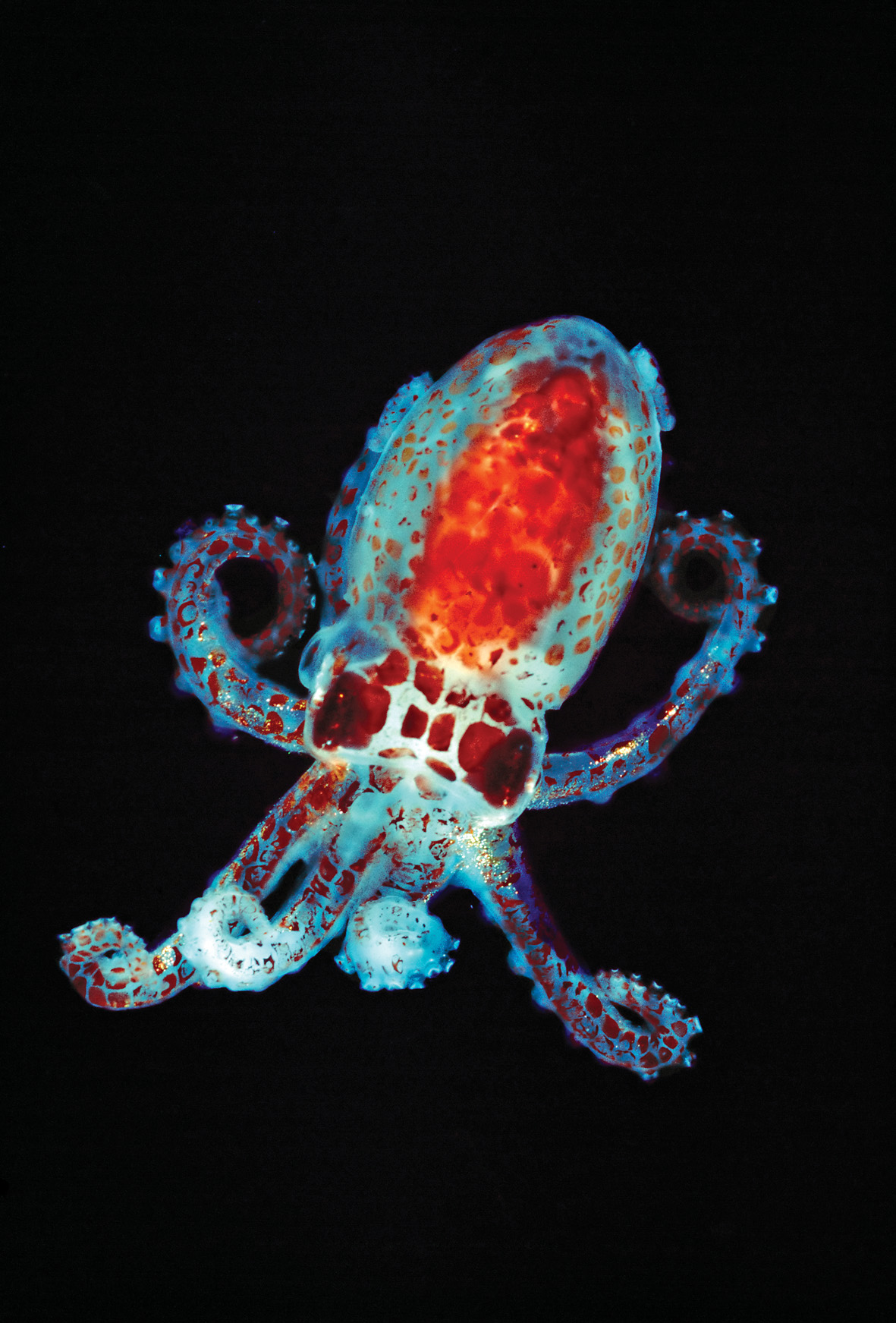
Symbiotic bacteria provide a variety of benefits to their hosts. Consider the bobtail squid, Euprymna scolopes, which possesses a specialized organ to house bacteria. The bacteria, Vibrio fischeri, are luminescent, that is, capable of generating light. The squid use their Vibrio-filled light organs like spotlights, projecting light downward to match light from the surface as a way to camouflage themselves from would-be predators below.
In many cases, animals and their bacterial symbionts are so intimately connected that it’s hard to separate one from the other. Many human gut bacteria are poorly studied because scientists haven’t been able to grow them outside the body. But some species have taken their intimate bacterial partnership a step further.
Aphids are small insects that feed on plant sap. They rely on the bacteria Buchnera aphidicola to synthesize certain amino acids for them that aren’t available from their plant-based diet. The partnership between aphids and Buchnera dates back as far as 250 million to 150 million years. Over time, the organisms have become completely dependent on one another for survival. The Buchnera bacteria actually live inside the aphid’s cells. In some ways, the bacteria resemble organelles rather than independent organisms. Intracellular symbionts may not be so unusual. In fact, mitochondria and chloroplasts—key organelles of eukaryotic cells—are thought to have originated from symbiotic bacteria. Over time, the progenitor bacteria became so intertwined with their host cells that they developed into an integral part of the cells themselves. Studying simple sapsucking aphids, it turns out, may offer insights into the evolutionary history of all eukaryotic cells.
Clearly, there are many good reasons to learn more about our bacterial comrades. From evolution to energy to human health, our microbiomes hold great promise—and great mystery. We’ve evolved hand in hand with these bacteria for millions of years. Let’s hope it takes less time to uncover their secrets.
Questions Arising from Case 5
Answers to Case 5 questions can be found in Chapters 26 and 27.
- How do intestinal bacteria influence human health? See page 26-22.
- What role did symbiosis play in the origin of chloroplasts? See page 27-4.
- What role did symbiosis play in the origin of mitochondria? See page 27-6.
- How did the eukaryotic cell originate? See page 27-7.