2.6 HOW DID THE MOLECULES OF LIFE FORM?
Case 1 The First Cell: Life’s Origins
In Chapter 1, we considered the similarities and differences between living and non-living things. Four billion years ago, however, the differences may not have been so pronounced. Scientists believe that life originated early in our planet’s history by a set of chemical processes that, through time, produced organisms that could be distinguished from their non-living surroundings. How can we think scientifically about one of biology’s deepest and most difficult problems? We can’t reconstruct key events from the geological record—the study of fossils tells us that life already existed when Earth’s oldest surviving fossil-containing rocks were deposited. The alternative is to approach life’s origins experimentally, asking whether chemical reactions likely to have taken place on the early Earth can generate the molecules of life. It is important to note that even the simplest living organisms living today are far more complicated than our earliest ancestors. No one suggests that cells as we know them emerged directly from primordial chemical reactions. Rather, the quest is to discover simple molecular systems able to replicate themselves, while subject to natural selection.
A key starting point is the observation, introduced earlier in this chapter, that the principal macromolecules found in organisms are themselves made of simpler molecules joined together. Thus, if we want to understand how proteins might have emerged on the early Earth, we should begin with the synthesis of amino acids, and if we are interested in nucleic acids, we should focus on nucleotides.
2-18
2.6.1 The building blocks of life can be generated in the laboratory.
Research into the origins of life was catapulted into the experimental age in 1953, with an elegant experiment carried out by Stanley Miller, then a graduate student in the laboratory of Nobel laureate Harold Urey. Miller started with gases such as water vapor, methane, and hydrogen gas, all thought to have been present in the early atmosphere. He put these gases into a sealed flask and then passed a spark through the mixture (Fig. 2.29). On the primitive Earth, lightning might have supplied the energy needed to drive chemical reactions, and the spark was meant to simulate its effects. Analysis of the contents of the flask showed that a number of amino acids were generated.
FIG. 2.29: Could the building blocks of organic molecules have been generated on the early Earth?
BACKGROUND In the 1950s, Earth’s early atmosphere was widely believed to have been rich in water vapor, methane, ammonia, and hydrogen gas, with no free oxygen.
EXPERIMENT Stanley Miller built an apparatus, shown below, designed to simulate Earth’s early atmosphere. Then he passed a spark through the mixture to simulate lightning.
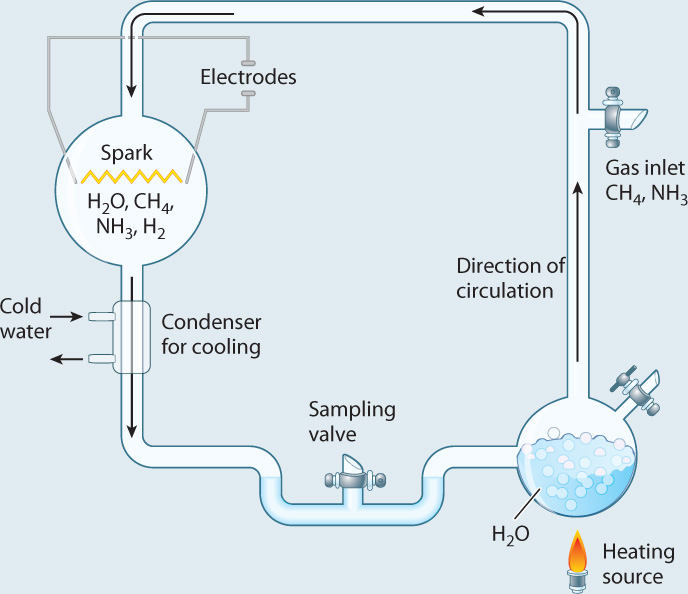
RESULTS As the experiment proceeded, reddish material accumulated on the walls of the flask. Analysis showed that the brown matter included a number of amino acids.
CONCLUSION Amino acids can be generated in conditions that mimic those of the early Earth.
FOLLOW-UP WORK Recent analysis of the original extracts, saved by Miller, shows that the experiment actually produced about 20 different amino acids, not all of them found in organisms.
SOURCE Miller, S. L. 1953. “Production of Amino Acids Under Possible Primitive Earth Conditions.” Science 117:528–529.
Miller and others conducted many variations on his original experiment, all with similar results. Today, many scientists doubt that the early atmosphere had the composition found in Miller’s experimental apparatus, but amino acids and other biologically important molecules can form in a variety of simulated atmospheric compositions. If oxygen gas (O2) is absent and hydrogen is more common in the mixture than carbon, the addition of energy generates diverse amino acids. The absence of oxygen gas is critical, since Miller-Urey-type reactions cannot run to completion in modern air or seawater. Here, however, geology supports the experiments: Chemical analyses of Earth’s oldest sedimentary rocks indicate that, for its first 2 billion years, Earth’s surface contained little or no oxygen.
Later experiments have shown that other chemical reactions can generate simple sugars, the bases found in nucleotides, and the lipids needed to form primitive membranes. Independent evidence that simple chemistry can form the building blocks of life comes from certain meteorites, which provide samples of the early solar system and contain diverse amino acids, lipids, and other organic compounds.
2.6.2 Experiments show how life’s building blocks can form macromolecules.
From the preceding discussion, we have seen that life’s simple building blocks can be generated under conditions likely to have been present on the early Earth—but can these simple units be stitched together to form the polymeric molecules of life? Once again, careful experiments have shown how polymers could have formed in the conditions of the early Earth. Clay minerals that form from volcanic rocks can bind nucleotides on their surfaces (Fig. 20.30a). The clays provide a surface that places the nucleotides in proximity to one another, making it possible for them to join to form chains, or simple strands of nucleic acid.
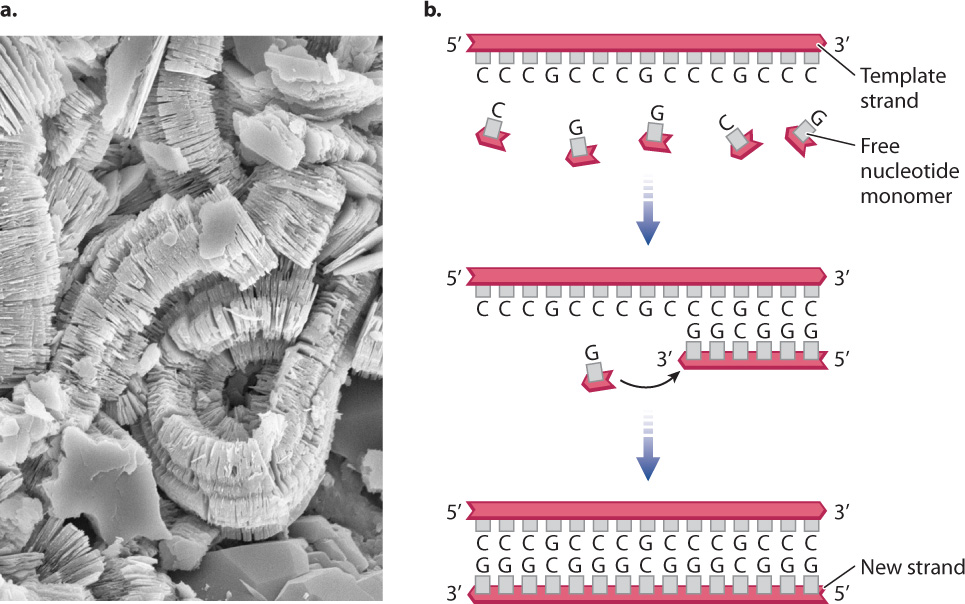
In a classic experiment, biochemist Leslie Orgel placed a short nucleic acid sequence into a reaction vessel and then added individual chemically modified nucleotides. The nucleotides spontaneously joined into a polymer, forming the sequence complementary to the nucleic acid already present (Fig. 20.30b).
2-19
Such experiments show that nucleic acids can be synthesized experimentally from nucleotide building blocks, but until recently the synthesis of nucleotides themselves presented a formidable problem for research on the origins of life. Many tried to generate nucleotides from their sugar, base, and phosphate constituents, but no one succeeded until 2009. That year, John Sutherland and his colleagues showed that nucleotides can be synthesized under conditions thought to be like those on the young Earth. These chemists showed how simple organic molecules likely to have formed in abundance on the early Earth react in the presence of phosphate molecules, yielding the long-sought nucleotides.
Such humble beginnings eventually gave rise to the abundant diversity of life we see around us, described memorably by Charles Darwin in the final paragraph of The Origin of Species:
It is interesting to contemplate an entangled bank, clothed with many plants of many kinds, with birds singing on the bushes, with various insects flitting about, and with worms crawling through the damp earth, and to reflect that these elaborately constructed forms, so different from each other, and dependent on each other in so complex a manner, have all been produced by laws acting around us.… There is grandeur in this view of life… from so simple a beginning endless forms most beautiful and most wonderful have been, and are being, evolved.