CHAPTER SUMMARY
4.1 PROTEINS ARE LINEAR POLYMERS OF AMINO ACIDS THAT FORM THREE-DIMENSIONAL STRUCTURES WITH SPECIFIC FUNCTIONS.
- An amino acid consists of an α carbon connected by covalent bonds to an amino group, a carboxyl group, a hydrogen atom, and a side chain or R group.
- There are 20 common amino acids that differ in their side chains.
- Amino acids are connected by peptide bonds to form proteins.
- The primary structure of a protein is its amino acid sequence. The primary structure determines how a protein folds, which in turn determines how it functions.
- The secondary structure of a protein results from the interactions of nearby amino acids. Examples include the α helix and β sheet.
- The tertiary structure of a protein is its three-dimensional shape, which results from long-range interactions of amino acid side chains.
- Some proteins are made up of several polypeptide subunits; this group of subunits is the protein’s quaternary structure.
- Chaperones help some proteins fold properly.
4.2 TRANSLATION IS THE PROCESS BY WHICH THE SEQUENCE OF BASES IN MESSENGER RNA SPECIFIES THE ORDER OF SUCCESSIVE AMINO ACIDS IN A NEWLY SYNTHESIZED PROTEIN.
- Translation requires many cellular components, including ribosomes, tRNAs, and proteins.
- mRNAs have three possible reading frames.
- tRNAs have an anticodon that base pairs with the codon in the mRNA and carries a specific amino acid.
- Aminoacyl tRNA synthetases attach specific amino acids to tRNAs.
- The genetic code defines the relationship between the three-letter codons of nucleic acids and their corresponding amino acids. It was deciphered using synthetic RNA molecules.
- The genetic code is redundant in that many amino acids are specified by more than one codon.
- Translation consists of three steps: initiation, elongation, and termination.
4.3 PROTEINS EVOLVE BY COMBINING FUNCTIONAL UNITS AND THROUGH MUTATION AND SELECTION.
- Protein families are groups of proteins that are structurally and functionally related.
- There are far fewer protein families than the total number of possible proteins because the probability that a random sequence of amino acids will fold properly to carry out a specific function is very small.
- A region of a protein that folds in a particular way and that carries out a specific function is called a folding domain.
- Proteins evolve by combining different folding domains.
- Changes in the amino acid sequence of a protein occur by mutation and selection.
Self-Assessment Question 1
Draw one of the 20 amino acids and label the amino group, the carboxyl group, the side chain (R group), and the α carbon.
Show Model Answer
Model Answer:
Briefly, the carboxyl group and α carbon are the same for all amino acids (see Fig. 4.2). The amino group is the same for almost all the amino acids, with Proline being the exception. The variety of function and form between the amino acids is mainly due to the different side chains (R groups).
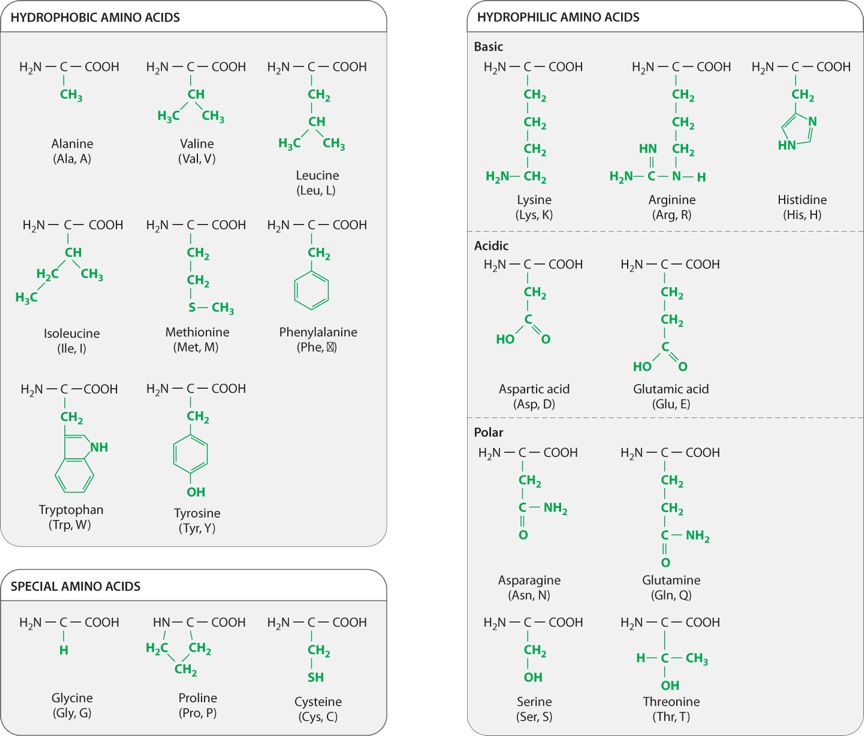
Self-Assessment Question 2
Name four major groups of amino acids, categorized by the properties of their side chains. Explain how the chemical properties of each group affect protein shape.
Show Model Answer
Model Answer:
Amino acids can be categorized into four main groups based on the properties of their side chains: The first are the hydrophobic amino acids, "water-fearing," whose side chains are non-polar, usually found buried in the interior of the folded proteins, and typically form bonds with other hydrophobic amino acids or solvents (e.g., Valine). In the second group are the hydrophilic amino acids, "water-loving," that have polar side chains, usually found on the outside surface of folded proteins, and typically form bonds with other hydrophilic amino acids or water. Their charge allows them to interact with other proteins and macromolecules. Hydrophilic amino acids are also broken up into two groups: basic amino acids with side chains that are positively charged at intracellular pH (e.g., Lysine) and acidic amino acids with side chains that are negatively charged (e.g., Aspartic acid).
Self-Assessment Question 3
Describe the importance to proteins of peptide bonds, hydrogen bonds, ionic bonds, disulfide bridges, and noncovalent interactions (e.g. van der Waals forces and the hydrophobic effect).
Show Model Answer
Model Answer:
The way in which amino acids interact and bond in a polypeptide chain is important for the structure and function of the protein.
Peptide bonds are important in maintaining primary structure of a polypeptide chain. These bonds form between the carboxyl group of one amino acid and the amino group of the next amino acid in the chain. Note that these bonds are typically found between groups in the polypeptide backbone.
Hydrogen bonds are important in maintaining secondary structure of the polypeptide chain. These bonds form between the oxygen in the carbonyl group of one peptide bond and the hydrogen in the amide group of another. This allows regions of the polypeptide to interact with itself and fold. Two common types of secondary structure formed by hydrogen bonding are α helices and β sheets (Figures 4.6 and 4.7). Note that in terms of secondary structure, these bonds are found between groups in the polypeptide backbone.
There are four groups of bonds or interactions important in creating tertiary and quaternary structure. The first group is the ionic bonds that form between a negative charge and a positive charge. For example, an ionic bond would form between a basic amino acid and an acidic amino acid because they have oppositely charged side chains. These bonds can occur between amino acids that are far apart in the polypeptide chain, thus creating loops and bends in the overall structure. Note that these bonds are typically found between side chains of the amino acids in the polypeptide backbone. The second group is the hydrogen bonds that form between the oxygen of one amino acid’s side chain and the hydrogen of another amino acid’s side chain. Note that when discussing tertiary structure, these bonds are found between different side chains. The third group is the disulfide bridges. These covalent bonds form between two cysteine residues in the same polypeptide chain, or between two cysteines in two different chains. The fourth group important to maintaining tertiary and quaternary structure is noncovalent interactions that include van der Waals forces and hydrophobic interactions that maintain interactions with different domains of the protein and results in a protein’s specific shape.
Self-Assessment Question 4
Explain how the order of amino acids determines the way in which a protein folds.
Show Model Answer
Model Answer:
The order of amino acids in the polypeptide chain determines the way in which proteins fold because of the various interactions and bonds formed between the amino acids. These interactions, depending on the type and location, will give rise to a specific secondary and tertiary structure. More often than not, these structures must be perfectly arranged for the protein to function. Thus, it is important that the polypeptide chain be correctly ordered to result in the specific structure of that particular protein.
Self-Assessment Question 5
Explain the relationship between protein folding and protein function.
Show Model Answer
Model Answer:
In many cases, the ability of a protein to perform its function is dependent upon the protein being in the correct confirmation. For example, many enzymes bind to their substrate through specific interactions. If a mutation causes an amino acid change in the gene resulting in the enzyme having a different shape, it may no longer be able to bind to its substrate and perform its activity.
Self-Assessment Question 6
Describe the relationship between codons of mRNA, anticodons of tRNA, and amino acids.
Show Model Answer
Model Answer:
The codons of mRNA are groups of three nucleotides that code for a particular amino acid. The sequence of the codons in the mRNA will give rise to the order of the resulting amino acid polypeptide chain. The codons are translated by tRNAs. Each tRNA has its own group of three nucleotides called an anticodon, that is complementary in sequence, and thus can recognize and bind to, a specific codon in the mRNA. Each tRNA also has a specific amino acid, affiliated with a particular anti-codon/codon pair, that is bound to the 3' end of the molecule. When the mRNA is being "read" through the ribosome, the order of the amino acids in the polypeptide chain is dependent upon the sequential interaction of the mRNA codon with the correct tRNA anticodon/amino acid pair. (See Figure 4.15)
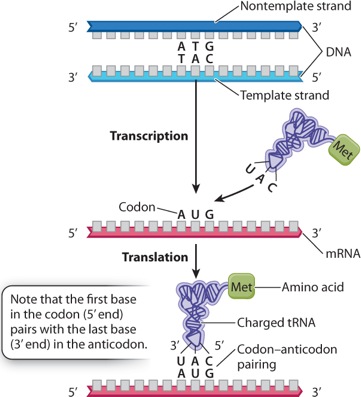
Self-Assessment Question 7
Describe the process by which ribosomes synthesize polypeptides.
Show Model Answer
Model Answer:
Translation of mRNA by ribosomes can be broken up into three processes:
Initiation: Initiation factors bind to the 5′ cap of the mRNA (in eukaryotic cells) or at the Shine-Dalgarno sequence (for prokaryotes) and recruit the small subunit of the ribosome and a tRNA charged with Methionine. This complex then moves along the mRNA until it finds a start codon (AUG, coding for Methionine). The large ribosomal subunit then joins the complex and causes the initiation factors to be released. The tRNAMet is then bound in the P site of the ribosome. The next tRNA, determined by the codon of the mRNA, binds in the A site of the ribosome. This elicits a coupled reaction in which the bond between the Met and its tRNA is broken and a new bond is formed between the carboxyl group of the Met and the amino group of the next amino acid (a peptide bond). The ribosome complex then slides to the next codon on the mRNA, shifting the now uncharged tRNAMet to the E site where it is released from the ribosome complex, and the peptide-bearing tRNA to the P site. The A site is now free for the next charged tRNA.
Elongation: The ribosome continues in this fashion shifting down the mRNA one codon at a time, adding amino acids to the growing peptide chain. Elongation factors provide the energy needed for these reactions to happen.
Termination: When the ribosome complex comes across a stop codon (UAA, UAG or UGA) a protein release factor binds in the A site of the ribosome and causes the bond between the polypeptide chain and the last tRNA to break. Once the polypeptide chain is released, the ribosomal subunits disassociate from the mRNA and each other and translation is complete.
Self-Assessment Question 8
Name and describe two ways that proteins can acquire new functions in the course of evolution.
Show Model Answer
Model Answer:
Two ways in which proteins can acquire new functions through the course of evolution are (1) mutation and selection and (2) combining different folding domains.
- Mutation and selection: The sequence of the amino acids in the polypeptide chain is important for the proper folding, and ultimately the function, of the protein. If the sequence is altered by a mutation that changes a codon to specify for a different amino acid, this could affect the function of the protein and whether it is selected for in the population. A mutation that leads to a non-functioning protein will most likely lead to the impaired survival and reproductive ability of that organism and will thus be eventually eliminated from the population. A neutral mutation that does not impair or improve protein function will likely remain in the population because those organisms will survive and reproduce at normal levels. A mutation that improves the function of the protein, although rare, would give a selective advantage to that organism if it could survive and reproduce more successfully.
- Combining different folding domains: form leads to function. If a gene gains a new folding domain by joining with a folding domain from another gene, for example, its product now has the additional function provided by that folding domain. If this function is beneficial or benign to the protein, and ultimately the survival and reproductive ability of the organism, the new gene, and therefore protein, will be maintained in the population.