5.1 STRUCTURE OF CELL MEMBRANES
Cells are defined by membranes. After all, membranes physically separate cells from their external environment. In addition, membranes define spaces within many cells that allow them to carry out their diverse functions. Lipids are the main component of cell membranes. Proteins are often embedded in or associated with the membrane. Carbohydrates can also be found in cell membranes, usually attached to lipids (glycolipids) and proteins (glycoproteins).
5-2
5.1.1 Cell membranes are composed of two layers of lipids.
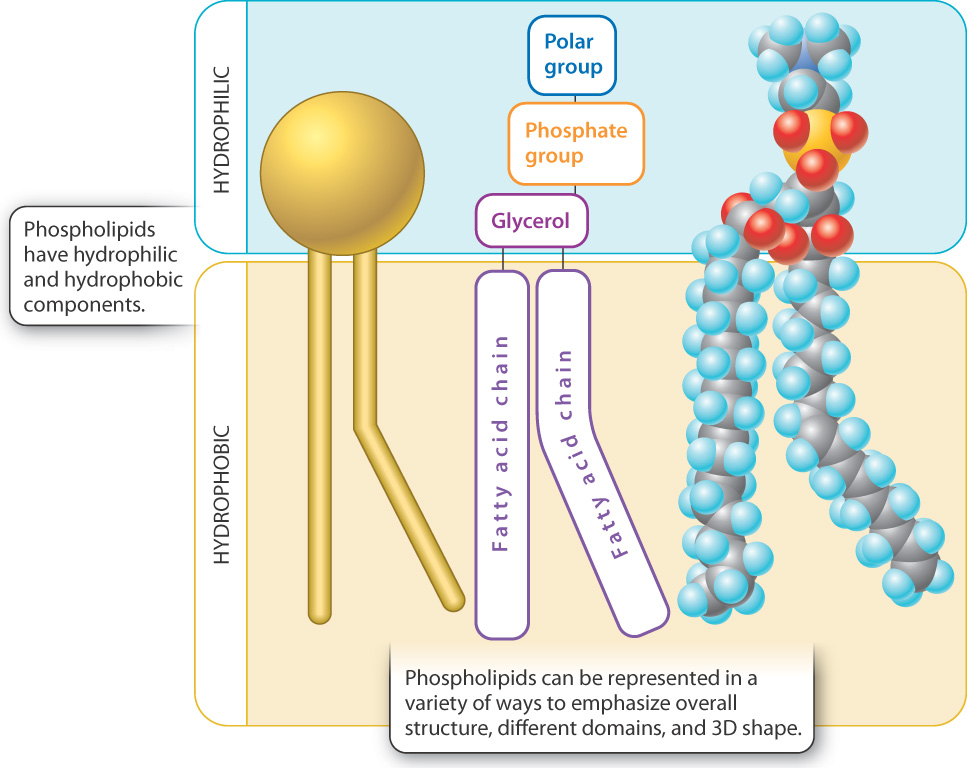
The major types of lipid found in cell membranes are phospholipids, introduced in Chapter 2. Phospholipids have both hydrophilic ("water-loving") and hydrophobic ("water-fearing") regions in a single molecule (Fig. 5.2). The phosphate "head" group is hydrophilic because it is polar, enabling it to form hydrogen bonds with water. By contrast, the two long fatty acid “tails” are hydrophobic because they are nonpolar and do not form hydrogen bonds with water. Molecules with both hydrophilic and hydrophobic regions are termed amphipathic.
In an aqueous environment, amphipathic molecules such as phospholipids behave in an interesting way. They spontaneously arrange themselves into various structures that have the polar head groups on the outside interacting with water and the nonpolar tail groups grouped together on the inside away from water. This arrangement results from the tendency of polar molecules like water to exclude nonpolar molecules or nonpolar groups within molecules. The shape of the structure is determined by the bulkiness of the head group relative to that of the hydrophobic tails. For example, lipids with bulky heads and a single hydrophobic fatty acid tail are wedge-shaped and pack into spherical structures called micelles (Fig. 5.3a). By contrast, lipids with less bulky head groups and two hydrophobic tails form a bilayer (Fig. 5.3b). A lipid bilayer is a two-layered structure organized in such a way that the hydrophilic portion of the lipid faces out toward the aqueous environment and the hydrophobic portion faces in toward the hydrophobic portions of other lipids and away from the aqueous environment.
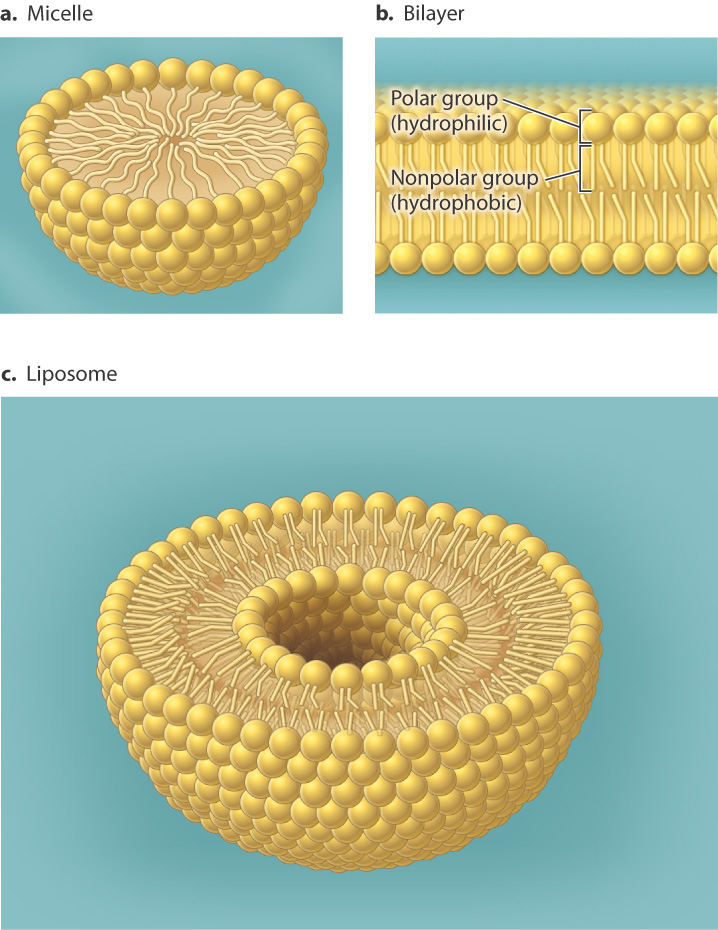
The resulting bilayers form closed structures with an inner space since free edges would expose the hydrophobic chains to the aqueous environment. This organization in part explains why bilayers are effective cell membranes. It also explains why membranes are "self-healing." Small tears in a membrane are rapidly sealed by a spontaneous rearrangement of the lipids surrounding the damaged region, due to the tendency of water to exclude nonpolar molecules.
5-3
5.1.2 How did the first cell membranes form?
Case 1 The First Cell: Life's Origins
We have seen that the phospholipid bilayer forms spontaneously in water, arranged in such a way that the hydrophobic fatty acids do not interact with water and the polar head groups do interact with water. The bilayer structure forms spontaneously as long as the concentration of free phospholipids is high enough and the pH of the solution is similar to that of a cell. The pH is important because it ensures that the head groups are in their ionized (charged) form and thus suitably hydrophilic. Thus, if phospholipids are added to a test tube of water at neutral pH, they spontaneously form enclosed bilayer structures called liposomes (Fig. 5.3c). An enzyme is not required to catalyze any chemical reaction. The membrane enclosure forms by a process of self-assembly that depends only on the chemical properties of the lipids. As the liposomes form, they may capture macromolecules present in solution.
Such a process may have been at work in the early evolution of life on Earth. Experiments show that liposomes can form and re-form in environments like tidal flats where wetting and drying occur repeatedly. The liposomes can even grow, incorporating more and more lipids from the environment. In addition, the liposomes can incorporate nucleic acids and other molecules into their interiors. Depending on their chemical composition, early membranes might have been either leaky or almost impervious to the molecules of life. Over time, they evolved in such a way as to allow at least limited molecular traffic between the environment and cell interior. At some point, new lipids no longer had to be incorporated from the environment. Instead, proteins guided lipid synthesis within the cell, although how this switch to synthesis happened remains uncertain.
All evidence suggests that membranes formed originally by straightforward physical processes, but that their composition and function evolved over time. François Jacob once said that evolution works more like a tinkerer than an engineer, modifying already existing materials rather than designing systems from scratch. It seems that the evolution of membranes is no exception to this pattern.
5.1.3 Cell membranes are dynamic.
Lipids freely associate with one another because of extensive van der Waals forces between their fatty acid tails (Chapter 2). These weak interactions are easily broken and re-formed, so lipid molecules are able to move within the plane of the membrane. For example, a single phospholipid can move across the entire length of a bacterial cell in less than a second. Lipids can also rapidly rotate around their vertical axis, and individual fatty acid chains are able to flex, or bend. As a result, membranes are dynamic, forming and re-forming continually during the lifetime of a cell.
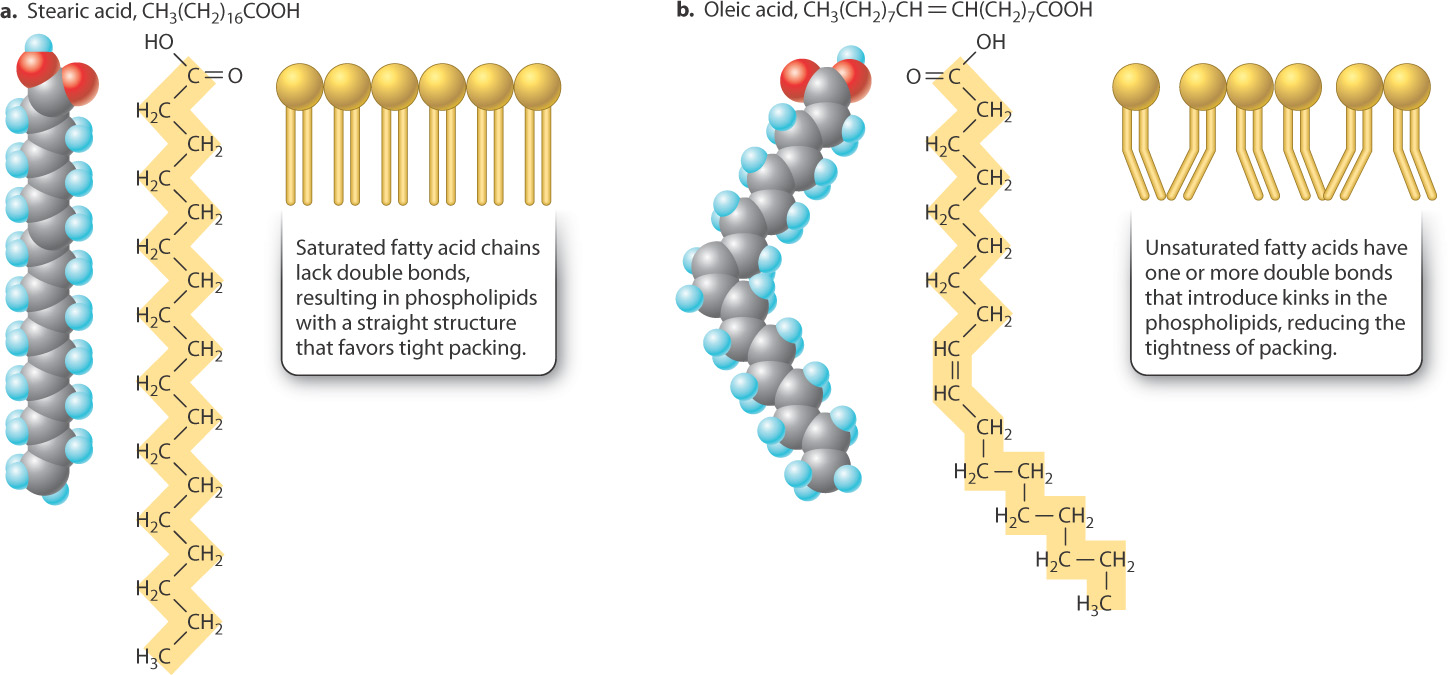
Because membrane lipids are able to move in the plane of the membrane, the membrane is said to be fluid. The degree of membrane fluidity depends on which types of lipid make up the membrane. In a single layer of the lipid bilayer, most of the van der Waals and hydrophobic interactions occur between the hydrophobic tails of lipids. The strength of these interactions depends on the length of the fatty acid tails and the presence of double bonds between neighboring carbon atoms. The longer the fatty acid tails, the more surface is available to participate in van der Waals interactions. The tighter packing that results tends to reduce lipid mobility. Likewise, saturated fatty acid tails, which have no double bonds, are straight and tightly packed—again reducing mobility (Fig. 5.4a). The double bonds in unsaturated fatty acids introduce kinks in the fatty acid tails, reducing the tightness of packing and enhancing lipid mobility in the membrane (Fig. 5.4b).
5-4
Question Quick Check 1
cMfKI7LsqkZBCvV0D8HzgPLn/rim3cbp71eLCscQwfl2Y/N6rfTPotssC3w9ZWMQRYa4S1oyUOwxkDvsdK4QVI/+L2MO05A0IH+9vy+6oAvsr2We3zn2EooOAD+mMrKI99ZFCSH+TEMnXtkaXNT8gl3ittO/F7rDsMOYutQX6oHvokiTXl5blH3GqxJe0859xHWs/fstOG8sJ8SB/Fg4wRz//7PsNf2Jf3F8cY1Xh3TeQi3XgGyRFPmO7jW7KdK4U5UHoRW2yyDfGC2MZAVGg82CUOUqscJz2V1xnHl0y8NbwrFb7b41oEhfP1UkGbyNbhRfcg==In addition to phospholipids, cell membranes often contain other types of lipid, and these can also influence membrane fluidity. For example, cholesterol is a major component of animal cell membranes, representing about 30% by mass of the membrane lipids. Like phospholipids, cholesterol is amphipathic, with both hydrophilic and hydrophobic groups in the same molecule. In the case of cholesterol, the hydrophilic region is simply a hydroxyl group (OH) and the hydrophobic region consists of four interconnected carbon rings with an attached hydrocarbon chain (Fig. 5.5). This structure allows cholesterol to insert into the lipid bilayer. Specifically, the hydrophilic head group of cholesterol interacts with the hydrophilic head group of phospholipids, while the ring structure participates in van der Waals interactions with the fatty acid chains.
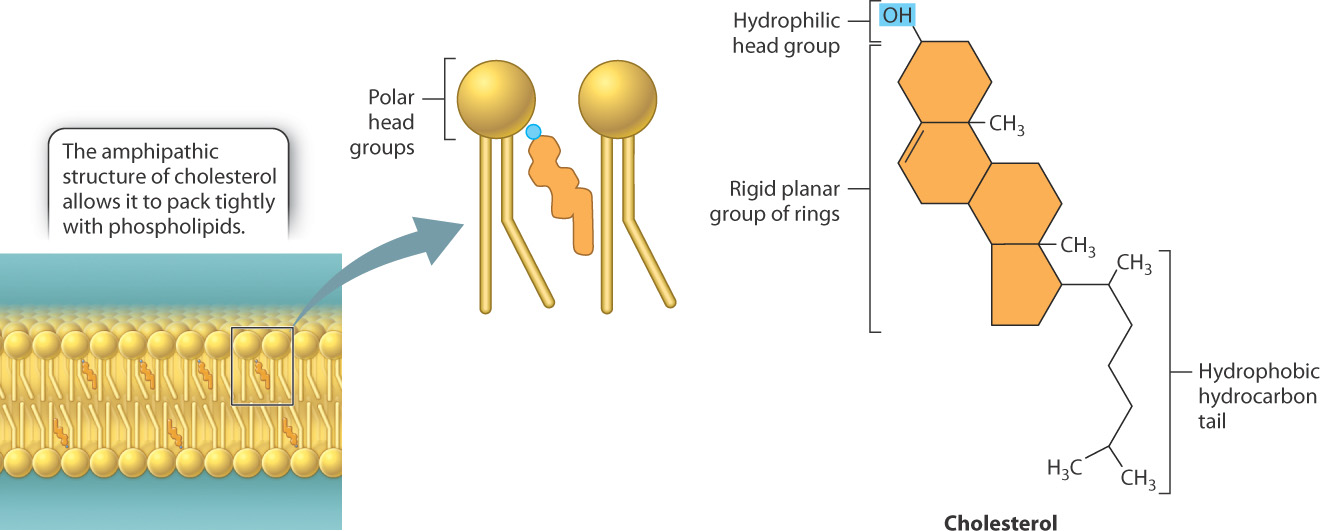
Cholesterol increases or decreases membrane fluidity depending on temperature. At normal temperatures typically found in a cell, the interaction of the rigid ring structure of cholesterol with the phospholipid fatty acid tails reduces the mobility of the phospholipids and hence the fluidity of the membrane. However, at low temperatures, cholesterol prevents phospholipids from packing tightly with other phospholipids and therefore increases membrane fluidity. Thus, cholesterol helps maintain a consistent state of membrane fluidity by preventing dramatic transitions from a fluid to solid state.
For many decades, it was thought that the various types of lipid found in the membrane were randomly distributed throughout the bilayer. However, more recent studies show that specific types of lipid sometimes assemble into defined patches called lipid rafts. For example, sphingolipids (a type of lipid) cluster in discrete patches in the membrane. Furthermore, cholesterol and other membrane components such as proteins appear to accumulate in some of these regions. Thus, membranes are not always a uniform fluid bilayer, but instead contain regions with discrete components.
Although lipids are free to move in the plane of the membrane, the spontaneous transfer of a lipid between layers of the bilayer, known as lipid flip-flop, is very rare. This is not surprising, since flip-flop requires the hydrophilic head group to pass through the hydrophobic interior of the membrane. As a result, there is little exchange of components between the two layers of the membrane, which in turn allows the two layers to differ in composition. In fact, in many membranes, different types of lipid are present primarily in one layer or the other.
5-5
5.1.4 Proteins associate with cell membranes in different ways.
Most membranes contain proteins as well as lipids. For example, the cell membrane of a red blood cell contains a large variety of proteins, representing as much as 50% of the membrane by mass. These membrane proteins serve different functions (Fig. 5.6). Some act as transporters, moving ions or other molecules across the membrane. Transporters include channels that allow movement of molecules through them, and carriers that facilitate movement. Other membrane proteins act as receptors that allow the cell to receive signals from the environment. Still others are enzymes that catalyze chemical reactions or anchors that attach to other proteins that help to maintain cell structure and shape.
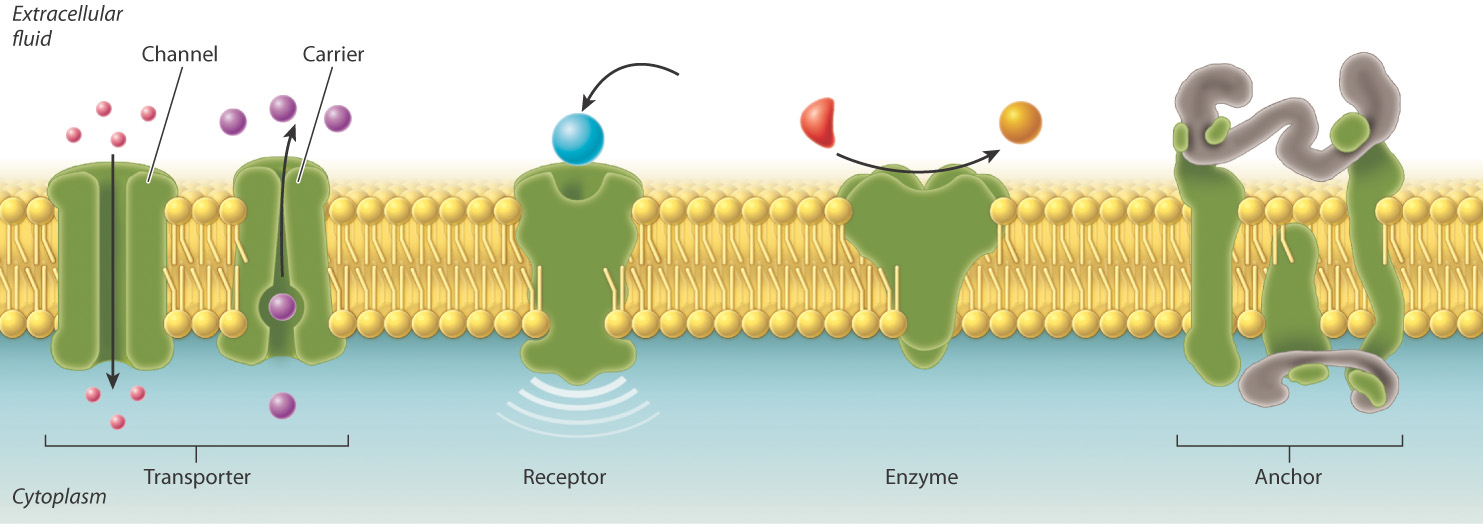
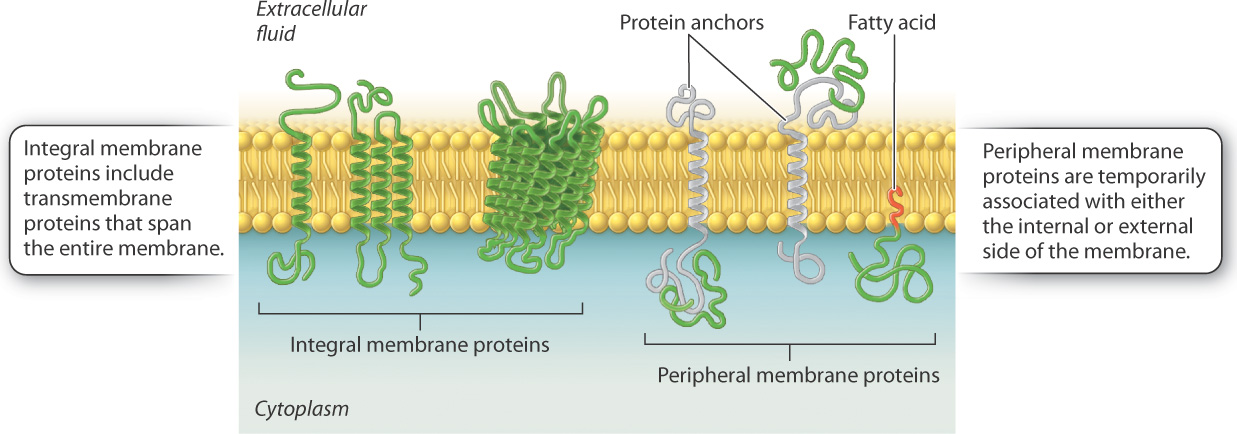
These various kinds of membrane protein can be classified into two groups depending on how they associate with the membrane (Fig. 5.7). Integral membrane proteins are permanently associated with cell membranes and cannot be separated from the membrane experimentally without destroying the membrane itself. Peripheral membrane proteins are temporarily associated with the lipid bilayer or with integral membrane proteins through weak noncovalent interactions. They are easily separated from the membrane by simple experimental procedures that leave the structure of the membrane intact.
5-6
Most integral membrane proteins are transmembrane proteins that span the entire lipid bilayer, as shown in Fig. 5.7. These proteins are composed of three regions: two hydrophilic regions, one protruding from each face of the membrane, and a connecting hydrophobic region that spans the membrane. The hydrophilic region on the internal side of the membrane often interacts with other proteins in the cytoplasm of the cell, whereas the hydrophilic region on the external side can interact with signaling molecules.
Peripheral membrane proteins may be associated with either the internal or external side of the membrane (Fig. 5.7). These proteins interact either with the polar heads of lipids or with integral membrane proteins via weak noncovalent interactions such as hydrogen bonds. Peripheral membrane proteins are only transiently associated with the membrane and can play a role in transmitting information received from external signals. Other peripheral membrane proteins limit the ability of transmembrane proteins to move within the membrane and assist proteins to cluster in lipid rafts.
Proteins, like lipids, are free to move in the membrane. How do we know this? The mobility of proteins in the cell membrane can be demonstrated using an elegant experimental technique called fluorescence recovery after photobleaching, or FRAP (Fig. 5.8). In this technique, proteins embedded in the cell membrane are labeled with fluorescent dye molecules. Labeling an entire membrane creates a fluorescent cell that can be visualized using a fluorescent microscope. A laser is then used to bleach the fluorescent dye molecules in a small area of the cell membrane, leaving a nonfluorescent spot on the surface of the cell. If proteins in the cell membrane were not capable of movement, the bleached area would remain nonfluorescent. However, over time fluorescence appears in the bleached area, telling us that fluorescent proteins that were not bleached moved into the bleached area.
FIG. 5.8: Do proteins move in the plane of the membrane?
BACKGROUND Fluorescent recovery after photobleaching (FRAP) is a technique used to measure mobility of molecules in the plane of the membrane. A fluorescent dye is attached to proteins embedded in the cell membrane in a process called labeling. A laser is then used to bleach a small area of the membrane.
HYPOTHESIS If membrane components such as proteins move in the plane of the membrane, the bleached spot should become fluorescent over time as unbleached fluorescent molecules move into the bleached area. If membrane components do not move, the bleached spot should remain intact.
EXPERIMENT AND RESULTS
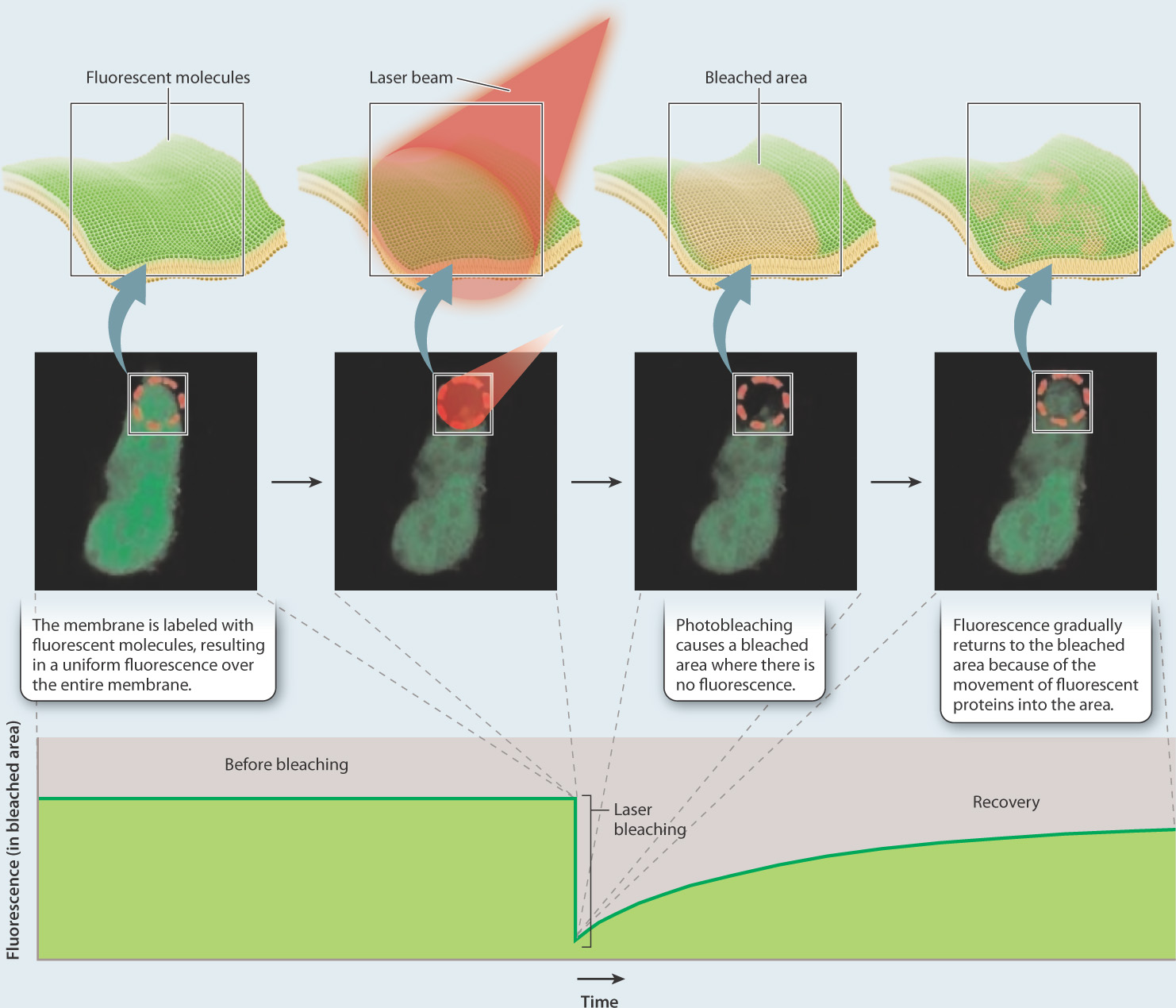
CONCLUSION The gradual recovery of fluorescence in the bleached area indicates that proteins move in the plane of the membrane.
SOURCE Peters, R., J. Peters, K.H. Tews, and W. Bähr. 1974. "A Microfluorimetric Study of Translational Diffusion in Erythrocyte Membranes." Biochim Biophys Acta 367: 282–294.
The idea that lipids and proteins coexist in the membrane, and that both are able to move in the plane of the membrane, led American biologists S. Jonathan Singer and Garth Nicolson to propose the fluid mosaic model in 1972. According to this model, the lipid bilayer is a fluid structure that allows molecules to move laterally within the membrane, and is a mosaic (a mixture) of two types of molecules, lipids and proteins.