5.4 THE ENDOMEMBRANE SYSTEM
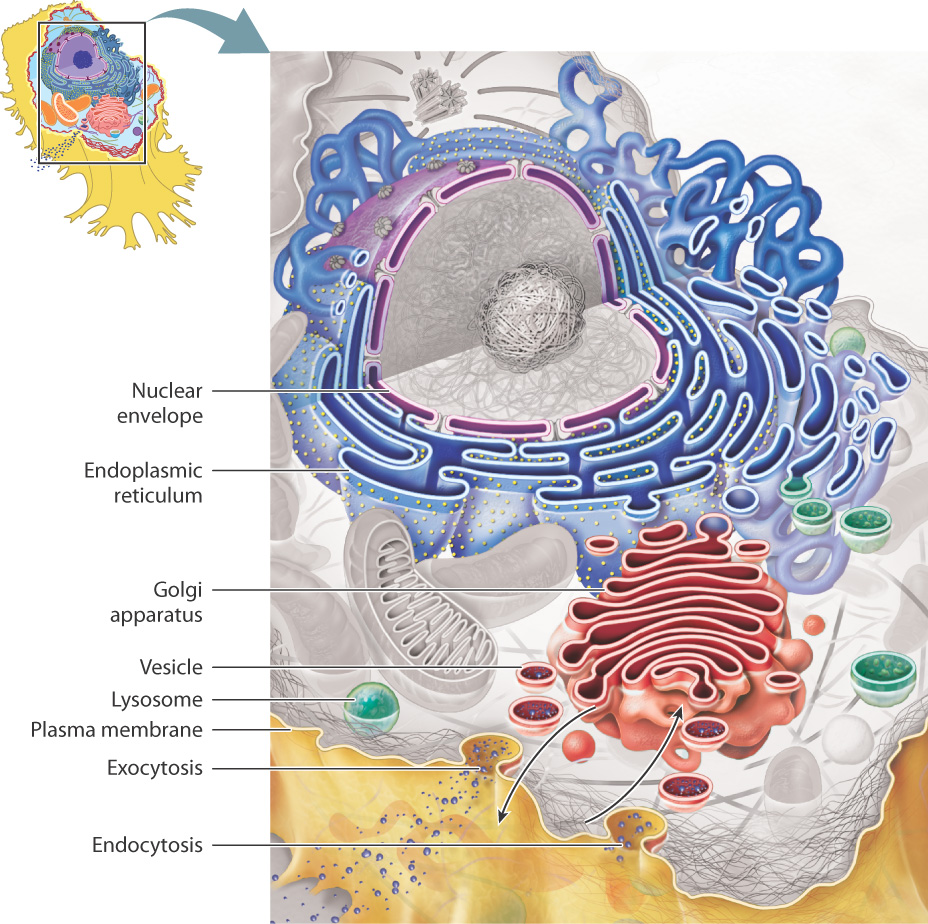
In eukaryotes, the total surface area of intracellular membranes is about tenfold greater than that of the plasma membrane. This high ratio of internal membrane area to plasma membrane area underscores the significant degree to which a eukaryotic cell is divided into internal compartments. Many of the organelles inside cells are not distinct, isolated entities, but instead communicate with one another. In fact, the membranes of these organelles are either physically connected by membrane "bridges" or communicate by the budding off and fusing of vesicles, small membrane-enclosed sacs that transport substances. In total, these membranes make up the endomembrane system. The endomembrane system includes the nuclear envelope, endoplasmic reticulum, Golgi apparatus, lysosomes, the plasma membrane, and the vesicles that move between them (Fig. 5.18).
5-16
Extensive internal membranes are not common in prokaryotic cells. However, photosynthetic bacteria do have internal membranes that are specialized for harnessing light energy (Chapters 8 and 26).
5.4.1 The endomembrane system compartmentalizes the cell.
Because of the selective permeability of cell membranes, the endomembrane divides the interior of a cell into two distinct "worlds," one inside the spaces defined by these membranes and one outside these spaces. Note that a molecule within the interior space of the ER can stay in the ER or end up within the Golgi apparatus or even outside the cell by the budding off and fusing of a vesicle between these organelles. Similarly, a molecule associated with the ER membrane can move to the Golgi membrane or the plasma membrane by vesicle transport. However, molecules in the cytosol are in a different physical space, separated by membranes of the endomembrane system. This physical separation allows specific functions to take place within the spaces defined by the membranes and also within the membrane itself.
In spite of forming a continuous and interconnected system, the various compartments have unique properties and maintain distinct identities determined by which lipids and proteins are present in their membranes. Recall that membranes are not fixed but dynamic, able to change their lipid and protein composition over time.
As we have seen, vesicles can bud off and fuse with components of the endomembrane system, creating a set of interconnected spaces. They can even fuse with the plasma membrane. This process, called exocytosis, provides a way for a vesicle to empty its contents to the extracellular space or to deliver proteins embedded in the vesicle membrane to the plasma membrane (Fig. 5.18). The process also works in reverse: A vesicle can bud off from the plasma membrane, bringing material from outside the cell into a vesicle, which can then fuse with other organelles. This process is called endocytosis. Together, exocytosis and endocytosis provide a way to move material into and out of cells without passing through the cell membrane.
5-17
5.4.2 The nucleus houses the genome and is the site of RNA synthesis.
The nucleus stores DNA, the genetic material that encodes the information necessary for all the activities and structures of the cell. The nuclear envelope defines the boundary of the nucleus (Fig. 5.19). It consists of two membranes, the inner and outer membranes, and each is a lipid bilayer with associated proteins.
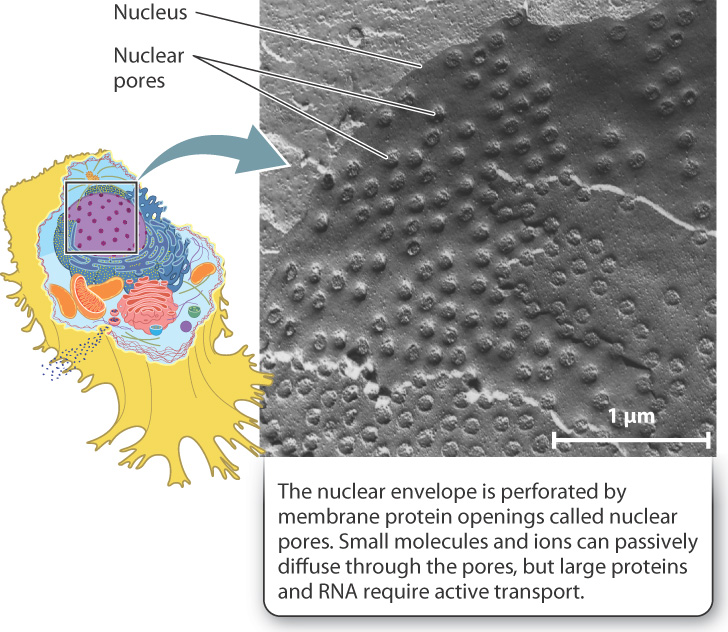
These two membranes are continuous with each other at protein openings called nuclear pores. These pores act as gateways that allow molecules to move into and out of the nucleus, and thus are essential for the nucleus to communicate with the rest of the cell. In fact, the transfer of information encoded by DNA depends on the movement of RNA molecules out of the nucleus, while the control of how and when this information is expressed depends on the movement of proteins into the nucleus. The nuclear envelope with its associated protein pores regulates which molecules move into and out of the nucleus.
5.4.3 The endoplasmic reticulum is involved in protein and lipid synthesis.
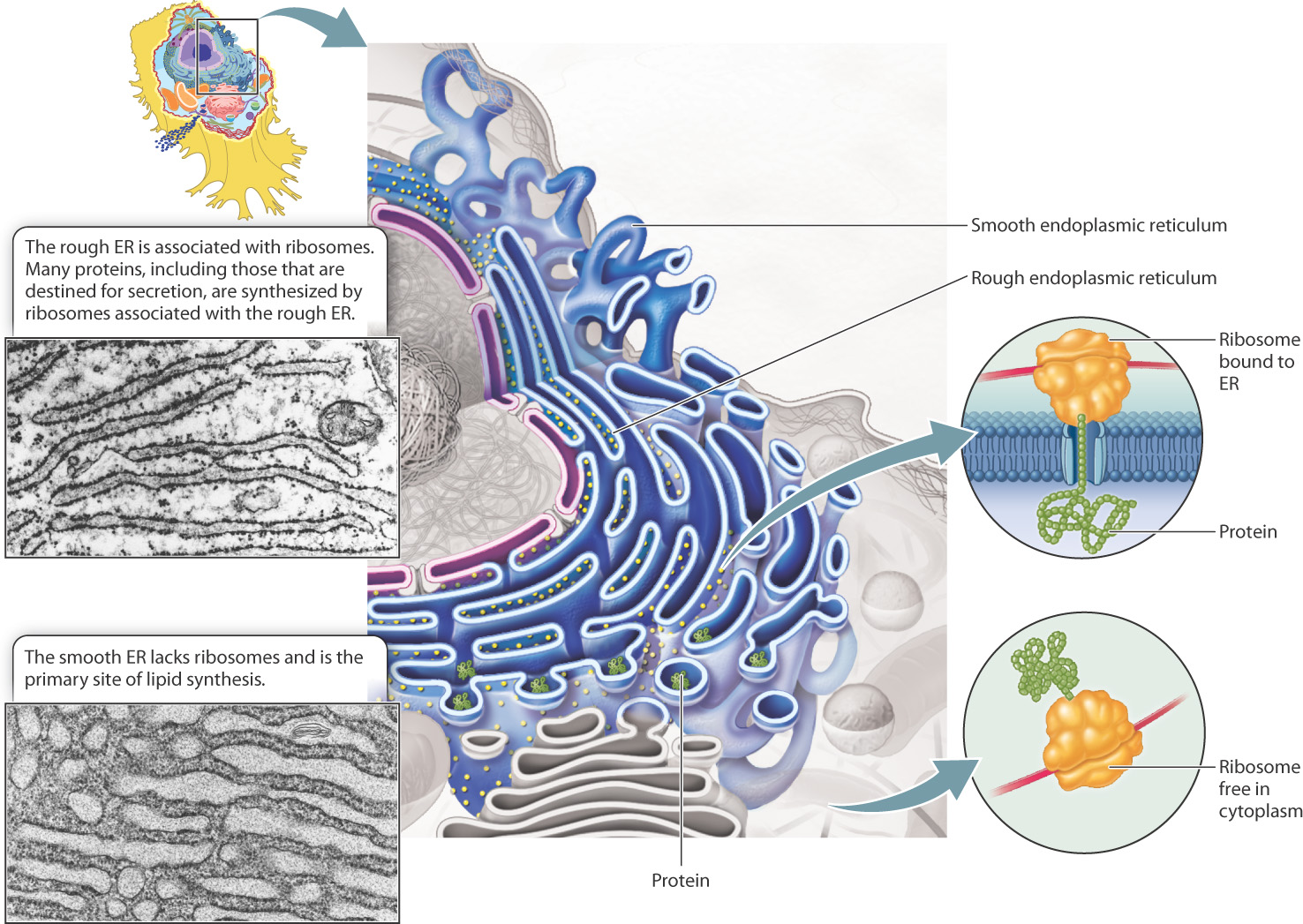
The outer membrane of the nuclear envelope is physically continuous with the endoplasmic reticulum (ER), an organelle bounded by a single membrane (Fig. 5.20). The ER is a conspicuous feature of many eukaryotic cells, accounting in some cases for as much as half of the total amount of membrane. The ER produces and transports many of the lipids and proteins used inside and outside the cell. It is the site of production of most of the lipids that make up the various cell membranes. In addition, transmembrane proteins and proteins destined for the Golgi apparatus, lysosomes, or export out of the cell are synthesized in the ER.
5-18
Unlike the nucleus, which is a single rounded structure in the cell, the ER consists of a complex network of interconnected tubules and flattened sacs. The interior of the ER is continuous throughout and is called the lumen. As shown in Fig. 5.20, the ER has an almost mazelike appearance when sliced and viewed in cross section. The ER membrane is extensively convoluted, allowing a large amount of membrane surface area to fit within the cell. The amount of ER membrane in a cell varies among cells of different functions. For example, mucus-producing cells of the gut synthesize a large amount of protein for export and thus have an extensive ER, as do the cells of the pancreas that produce the protein insulin. In cells that do not secrete large quantities of protein, the ER can be quite small.
When viewed through an electron microscope, ER membranes have two different appearances (Fig. 5.20). In most cells, the majority of ER membranes have small, rounded particles associated with them that are exposed to the cytosol. This portion of the ER is referred to as rough endoplasmic reticulum (RER). The particles bound to the cytosolic face of the RER are ribosomes. Ribosomes are the sites of protein synthesis, in which amino acids are assembled into polypeptides guided by the information stored in mRNA (Chapter 4). Ribosomes can be free in the cytosol or associated with the ER membrane.
In most cells, there is a small amount of ER membrane that lacks ribosomes and is consequently called smooth endoplasmic reticulum (SER) (Fig. 5.20). Portions of the smooth ER membrane actively bud off to produce vesicles that are free to move in the cytosol. Since each vesicle is formed from a patch of ER membrane and encloses a portion of the ER lumen, vesicles are an effective means of moving proteins that are either embedded in the ER membrane or free floating inside.
SER is also the site of fatty acid and phospholipid biosynthesis. Thus, this type of ER predominates in cells specialized for the production of lipids. For example, cells that synthesize steroid hormones have a well-developed SER that produces large quantities of cholesterol and contains enzymes that convert cholesterol into steroid hormones.
5.4.4 The Golgi apparatus modifies and sorts proteins and lipids.
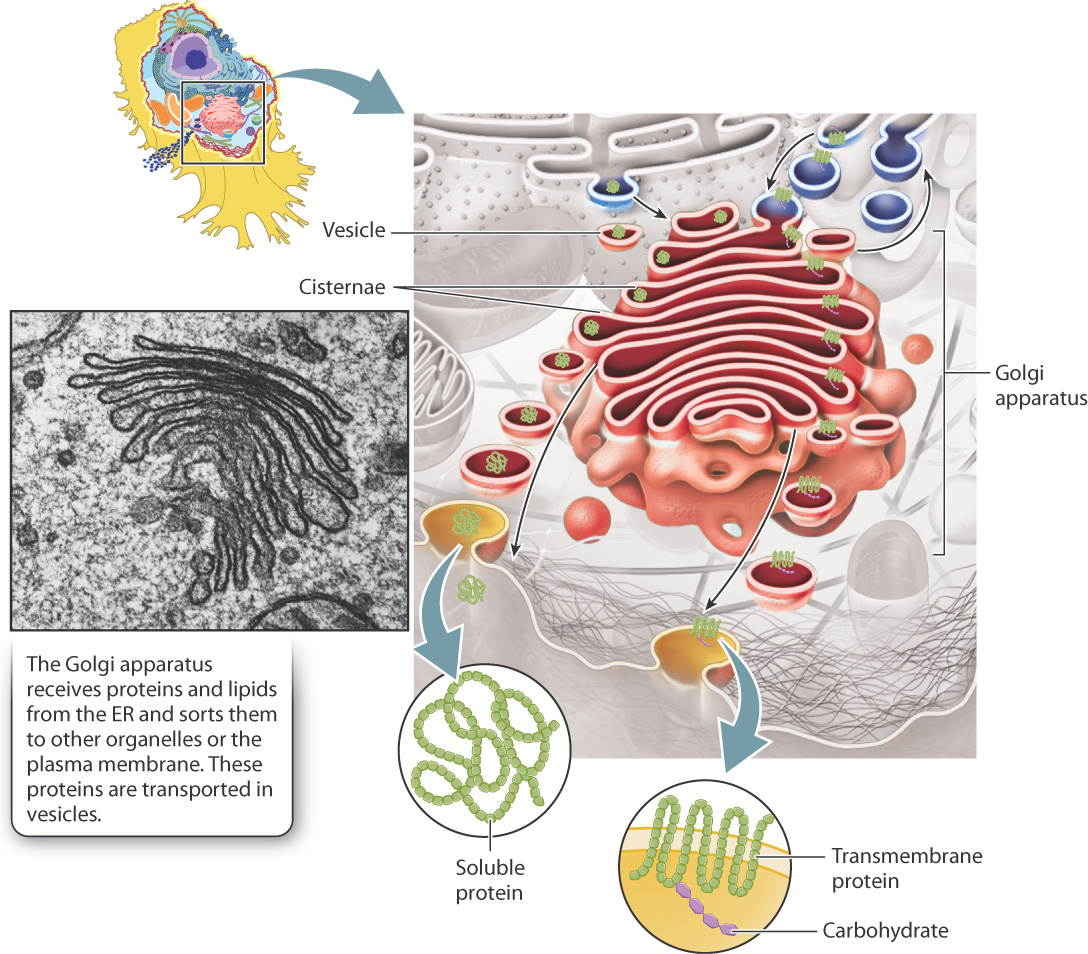
Although it is not physically continuous with the ER, the Golgi apparatus is often the next stop for vesicles that bud off the ER. These vesicles carry lipids and proteins that are either located in the vesicle interior or embedded in their membranes. The movement of these vesicles from the ER to the Golgi apparatus and then to the rest of the cell is part of a biosynthetic pathway in which lipids and proteins are successively modified and delivered to their final destinations. The Golgi apparatus has three primary roles: (1) It further modifies proteins and lipids produced by the ER; (2) it acts as a sorting station as they move to their final destinations; and (3) it is the site where most of the cell’s carbohydrates are synthesized.
Under the microscope, the Golgi apparatus looks like a series of flattened membrane sacs, which are called cisternae (Fig. 5.21). The cisternae, which are stacked, are surrounded by many small vesicles. These vesicles transport proteins from the ER to the Golgi apparatus, between the various cisternae, and between the Golgi apparatus and the plasma membrane or other organelles. Vesicles are therefore the primary means by which proteins and lipids move through the Golgi apparatus to their final destinations.
5-19
Enzymes within the Golgi apparatus chemically modify proteins and lipids as they pass through it. These modifications are sequential since each region of the Golgi apparatus contains a different set of enzymes that catalyze specific reactions. As a result, there is a general movement of vesicles from the ER through the Golgi apparatus and then to their final destinations.
An example of a chemical modification that occurs predominantly in the Golgi apparatus is glycosylation, in which sugars are covalently linked to lipids or specific amino acids of proteins. As these lipids and proteins move through the Golgi apparatus, sugars are added and trimmed in a sequential fashion. Glycoproteins are important components of the eukaryotic cell surface because their attached sugars can protect the protein from enzyme digestion by blocking access to the peptide chain. As a result, glycoproteins form a relatively flexible and protective coating over the plasma membrane. The distinctive shapes that sugars contribute to glycoproteins and glycolipids also allow cell surface components to be recognized specifically by other cells and molecules in the external environment. For example, human blood types (A, B, AB, and O) are distinguished by the particular sugars that are linked to proteins and lipids on the surface of red blood cells.
While traffic usually travels from the ER to the Golgi apparatus, there is also a small amount of traffic moving in the reverse direction, from the Golgi apparatus to the ER. This reverse pathway is important to retrieve ER or Golgi resident proteins that are accidentally moved forward, and to recycle membrane components.
5.4.5 Lysosomes degrade macromolecules.
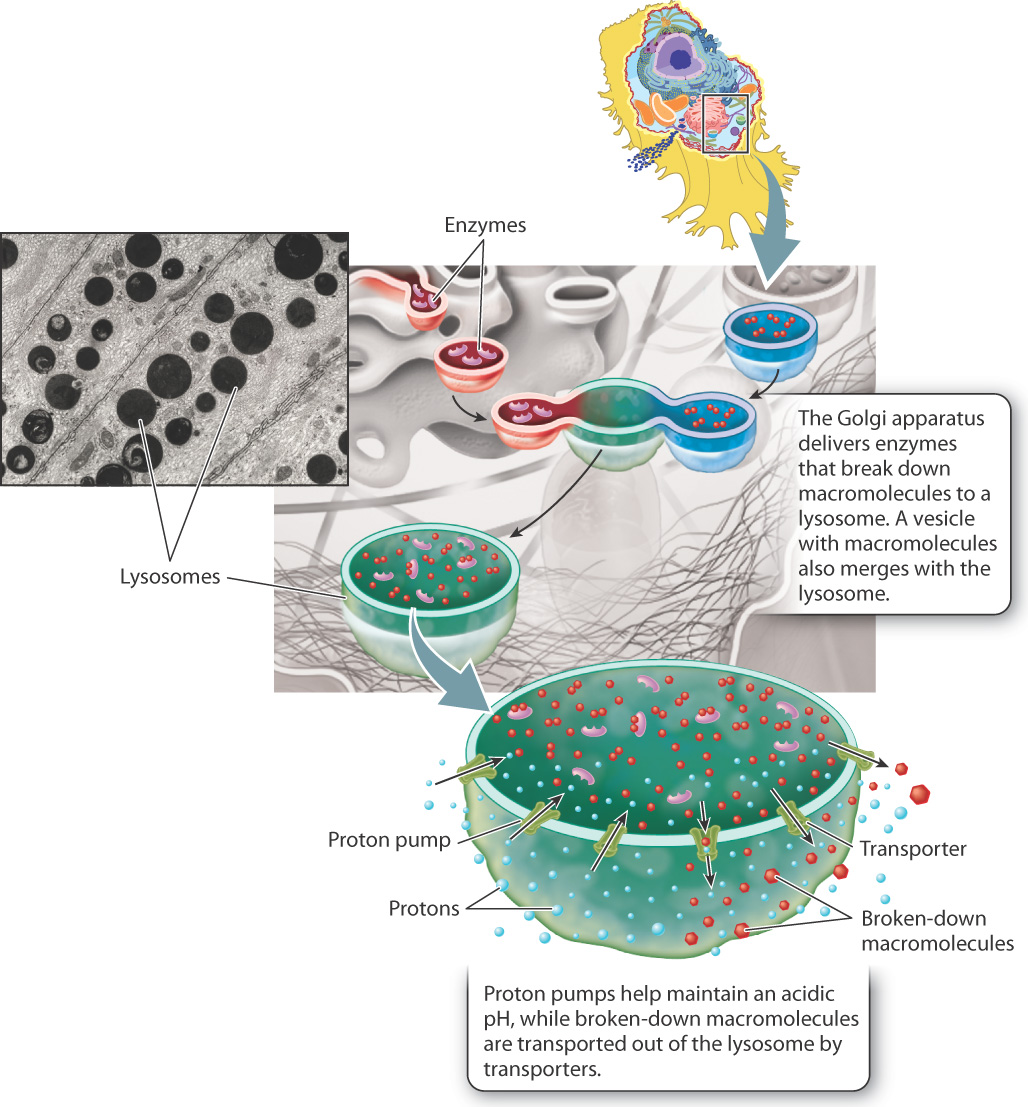
The ability of the Golgi apparatus to sort and dispatch proteins to particular destinations is dramatically illustrated by lysosomes. Lysosomes are specialized vesicles derived from the Golgi apparatus that degrade damaged or unneeded macromolecules. They contain a variety of enzymes that break down macromolecules such as proteins, nucleic acids, lipids, and complex carbohydrates (Fig. 5.22). Macromolecules destined for degradation are packaged by the Golgi apparatus into vesicles. The vesicles then fuse with lysosomes, delivering their contents to the lysosome interior.
The formation of lysosomes also illustrates the ability of the Golgi apparatus to sort key proteins. The enzymes inside of lysosomes are synthesized in the RER, sorted in the Golgi apparatus, and then packaged into lysosomes. In addition, the Golgi apparatus sorts and delivers specialized proteins that become embedded in lysosome cell membranes. These include proton pumps that keep the internal environment at an acidic pH of around 5, the optimum pH for the activity of the enzymes inside. Other proteins in the lysosomal membranes transport the breakdown products of macromolecules, such as amino acids and simple sugars, across the membrane to the cytosol for use by the cell.
The function of lysosomes underscores the importance of having separate compartments within the cell bounded by selectively permeable membranes. Many of a cell’s enzymes and proteins would unfold and degrade if the entire cell were at a pH of 5, and lysosomal enzymes cannot function in the normal cellular environment, which has a pH of about 7. By restricting the activity of these enzymes to the lysosome, proteins and organelles in the cytosol are protected from degradation.
5-20
5.4.6 Protein sorting directs proteins to their proper location in or out of the cell.
As we have seen, eukaryotic cells have many compartments, and different proteins function in different places, such as enzymes that act in lysosomes or transmembrane proteins embedded in the plasma membrane. Protein sorting is the process by which proteins end up where they need to be to perform their function. Protein sorting directs proteins to the cytosol, the lumen of organelles, the membranes of the endomembrane system, or even out of the cell entirely. Let’s consider in more detail how proteins produced from free ribosomes and those produced from membrane-bound ribosomes on the RER are sorted to their final destinations in the cell.
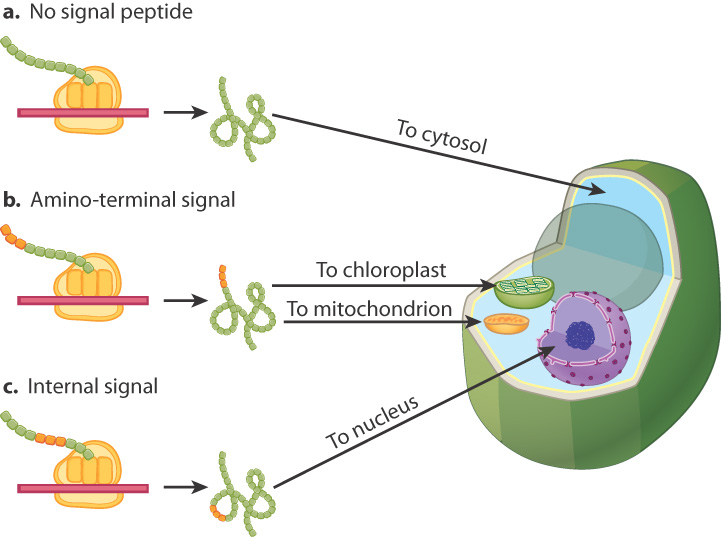
Proteins produced on free ribosomes start off in the cytosol and are sorted to their final destination after translation. These proteins are often directed to their proper cellular compartments by means of particular amino acid sequences called signal sequences. As shown in Fig. 5.23, there are several types of signal sequences that direct proteins to different cellular compartments. Proteins with no signal sequence remain in the cytosol (Fig. 5.23a). Most proteins with a signal sequence at their amino ends are targeted to mitochondria or chloroplasts (Fig. 5.23b). Proteins targeted to the nucleus usually have signal sequences located internally (Fig. 5.23c). The signal sequence for the nucleus, also called a nuclear localization signal, enables proteins to move through pores in the nuclear envelope.
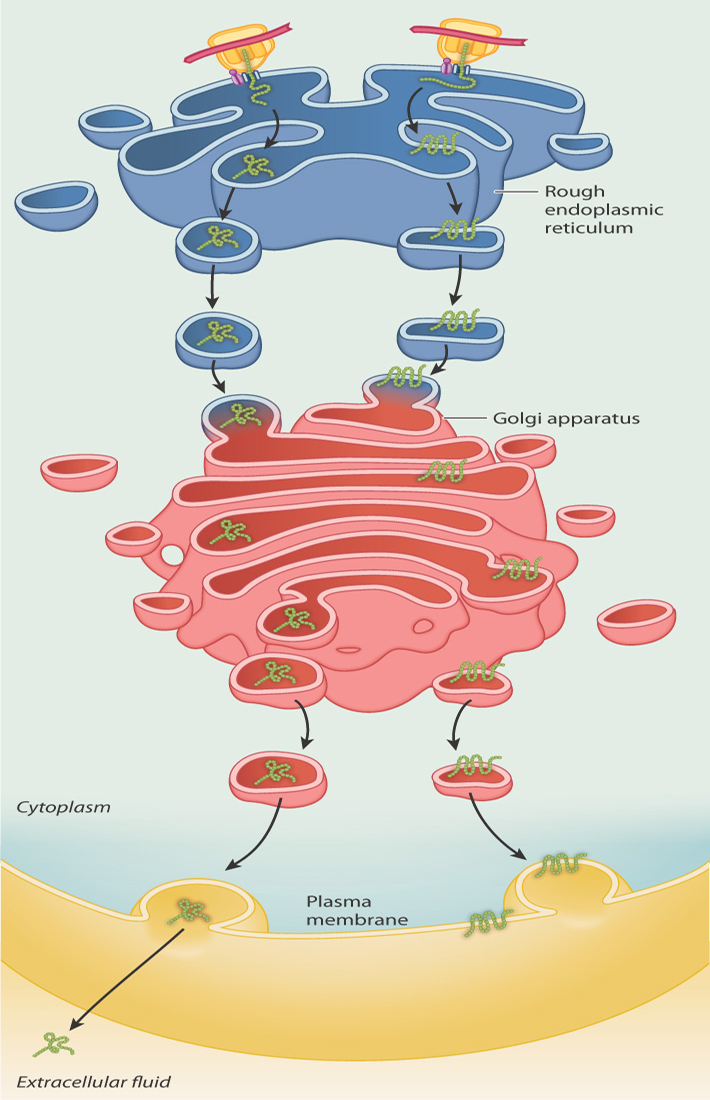
Proteins produced by ribosomes on the RER end up within the lumen of the endomembrane system or embedded in its membrane, or they may be secreted out of the cell. These proteins are sorted as they are translated: Proteins destined for the lumen or for secretion are fed into the ER lumen as they are synthesized, whereas the proteins destined for membranes are inserted in the ER membrane as they are synthesized (Fig. 5.24).
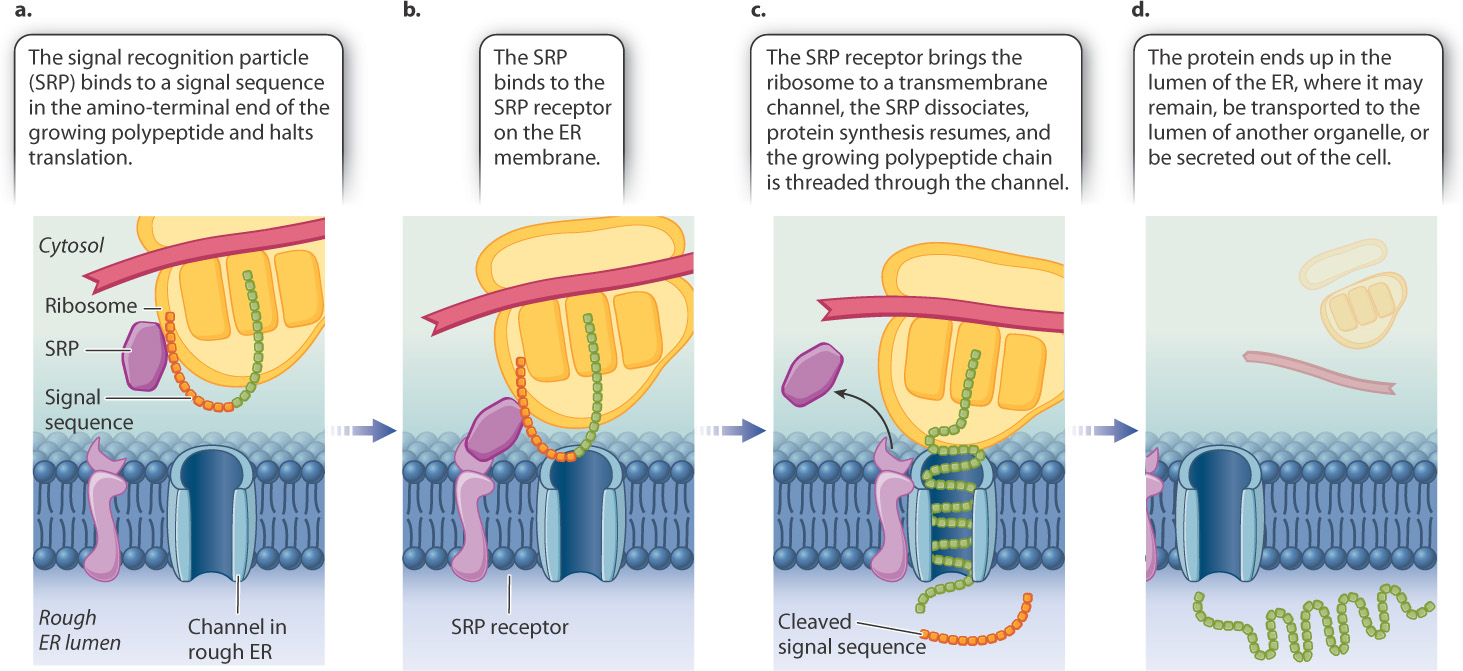
The polypeptide chains of proteins can move into the lumen or membrane because of the presence of an amino-terminal signal sequence shown in Fig. 5.25. This signal sequence is recognized almost immediately after synthesis by an RNA–protein complex known as a signal-recognition particle (SRP). The SRP binds to both the signal sequence and the ribosome and brings about a pause in translation (Fig. 5.25a). The SRP then binds with a receptor on the RER (Fig. 5.25b). The SRP dissociates and translation continues. The SRP receptor brings the ribosome to a channel through the hydrophobic membrane of the RER. Each growing polypeptide chain is threaded through the channel (Fig. 5.25c). A specific protease cleaves the signal sequence as it emerges in the lumen of the ER. The finished protein ends up in the lumen of the RER (Fig. 5.25d).
5-21
Whether the polypeptide chain ends up in the membrane or in the lumen depends on whether or not the polypeptide chain has a signal-anchor sequence in its interior. Proteins destined for the lumen of the ER, the Golgi apparatus, a lysosome, or the exterior of the cell have no signal-anchor sequence. These chains are fed completely through the channel into the lumen. Then, chaperone proteins assist with protein folding. Some proteins are retained in the ER, while others are transported in vesicles to the Golgi apparatus. Some of these proteins end up being secreted by exocytosis, as shown on the left in Fig. 5.24.
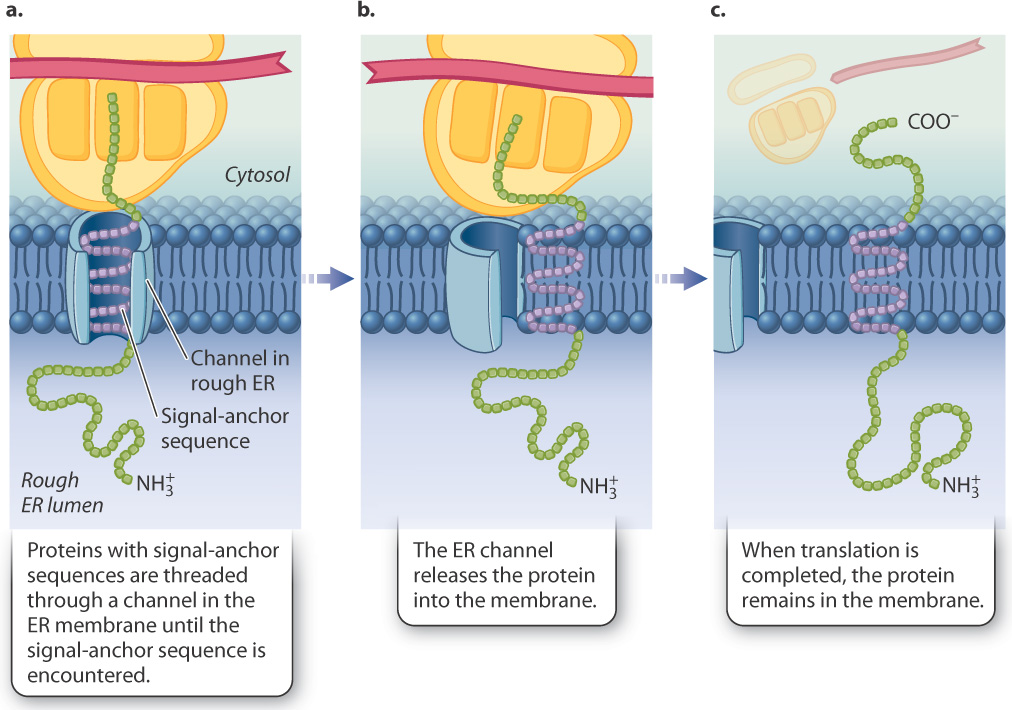
Proteins destined to be embedded in membranes (shown on the right in Fig. 5.24) do contain signal-anchor sequences (Fig. 5.26). The growing polypeptide chain is transported across the channel in the ER membrane until the signal-anchor sequence is encountered (Fig. 5.26a). In the case of transmembrane proteins, the signal-anchor sequence is hydrophobic and can become so intimately associated with the membrane that it exits the channel and diffuses laterally in the lipid bilayer (Fig. 5.26b). At this point, the ribosome disassociates from the channel while translation continues (Fig. 5.26c). When translation is completed and the polypeptide is released, the carboxyl end of the chain remains on the cytosolic side of the ER membrane, the amino end is in the ER lumen, and the membrane-bound region between them anchors both sides to the membrane. Transmembrane proteins such as these may stay in the membrane of the ER or end up in other internal membranes or the plasma membrane, where they serve as channels, pumps, receptors, or enzymes.
5-22