6.1 AN OVERVIEW OF METABOLISM
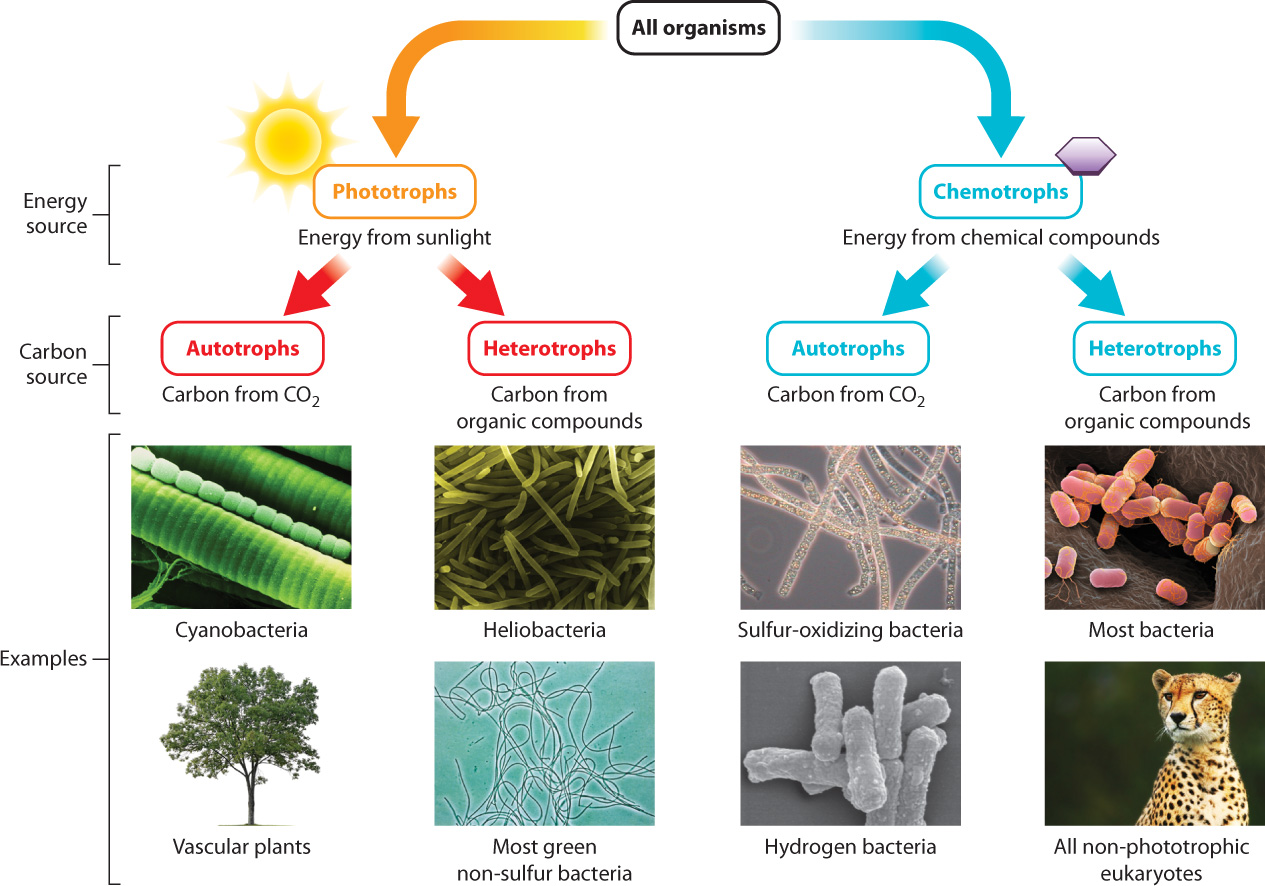
When considering a cell’s use of energy, it is also useful to consider the cell’s sources of carbon, because carbon is the backbone of the organic molecules that make up cells and because cells often use carbon-based compounds as a source of energy to carry out their functions. How organisms obtain the energy and carbon needed for growth and other vital functions is so fundamental that it is sometimes used to provide a metabolic classification of life (Fig. 6.1). Simply put, organisms have two ways of harvesting energy from their environment and two sources of carbon. Together, this means that there are four principal ways in which organisms acquire the energy and materials needed to grow, function, and reproduce.
6-2
6.1.1 Organisms can be classified according to their energy and carbon sources.
Organisms have two ways of harvesting energy from their environment: They can obtain energy either from the sun or from chemical compounds (Fig. 6.1). Organisms that capture energy from sunlight are called phototrophs. Plants are the most familiar example. Plants use the energy of sunlight to convert carbon dioxide and water into sugar and oxygen (Chapter 8). Sugars, such as glucose, contain energy in their chemical bonds that is used to synthesize ATP, which in turn can power the work of the cell.
Other organisms derive their energy directly from organic molecules such as glucose. These organisms are called chemotrophs, and animals are familiar examples. Animals ingest other organisms, obtaining organic molecules such as glucose that they break down in the presence of oxygen to produce carbon dioxide and water. In this process, the energy in the organic molecule is converted to energy carried in the bonds of ATP (Chapter 7).
In drawing this distinction between plants and animals, we have to be careful: Although sunlight provides the energy that plants use to synthesize glucose, plants still power most cellular processes by breaking down the sugar they make, just as animals do. And although chemotrophs harness energy from organic molecules, the energy in these organic molecules is generally derived from the sun. Bearing these two points in mind, we use the terms “phototroph” and “chemotroph” because they call our attention to the flow of energy from the sun to organisms and then from one organism to the next (discussed more fully in Chapter 25).
Organisms can also be considered in terms of their source of carbon (Fig. 6.1). Some organisms are able to convert carbon dioxide (an inorganic form of carbon) into glucose (an organic form of carbon). These organisms are autotrophs, or “self feeders,” because they make their own organic sources of carbon. Plants again are an example, so plants are both phototrophs and autotrophs, or photoautotrophs.
Other organisms do not have the ability to convert carbon dioxide into organic forms of carbon. Instead, they obtain their carbon from organic molecules synthesized by other organisms (called preformed organic molecules)—that is, they eat other organisms or molecules derived from other organisms. Such organisms are heterotrophs, or “other feeders,” as they rely on other organisms for their organic source of carbon. Animals obtain their carbon in this way, and so animals are both chemotrophs and heterotrophs, or chemoheterotrophs.
6-3
As we move away from the familiar examples of plants and animals and begin in Part 2 to explore the diversity of life, we will see that not all organisms fit into these two categories (Fig. 6.1). Some microorganisms gain energy from sunlight, but obtain their carbon from preformed organic molecules; such organisms are called photoheterotrophs. Other microorganisms extract energy from inorganic sources but build their own organic molecules; these organisms are termed chemoautotrophs. They are often found in extreme environments, such as deep-sea vents, where sunlight is absent and inorganic compounds such as hydrogen sulfide are plentiful.
6.1.2 Metabolism is the set of chemical reactions that sustain life.
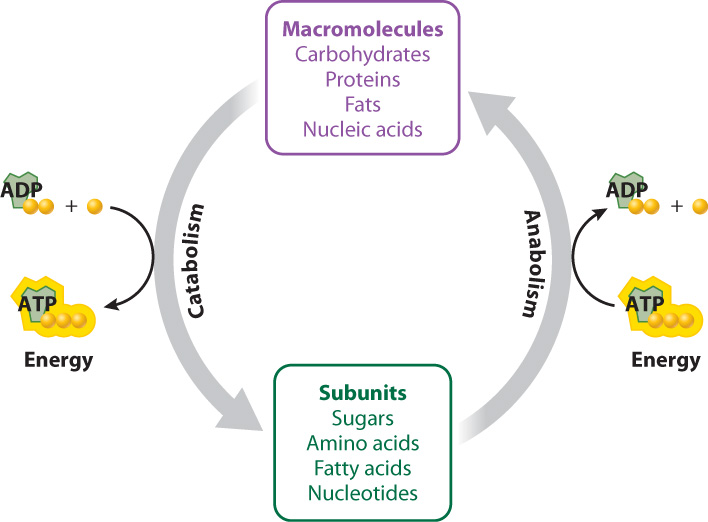
The building and breaking down of sugars such as glucose, and the harnessing and release of energy in the process, are driven by chemical reactions in the cell. The term metabolism encompasses the entire set of these chemical reactions that convert molecules into other molecules and transfer energy in living organisms. These chemical reactions are occurring all of the time in your cells. Many of these reactions are linked, in that the products of one are the reactants of the next, forming long pathways and intersecting networks.
Metabolism is divided into two branches: Catabolism is the set of chemical reactions that break down molecules into smaller units and, in the process, produce ATP, and anabolism is the set of chemical reactions that build molecules from smaller units and require an input of energy, usually in the form of ATP (Fig. 6.2). For example, carbohydrates can be broken down, or catabolized, into sugars, fats into fatty acids and glycerol, and proteins into amino acids. These initial products can be broken down further to release energy stored in their chemical bonds. The synthesis of macromolecules such as sugars and proteins, by contrast, is anabolic.