6.3 THE LAWS OF THERMODYNAMICS
Energy from the sun can be converted by plants and other phototrophs into chemical potential energy held in the bonds of molecules. Chemical reactions can transfer this chemical energy between different molecules. And energy from these molecules can be used by the cell to do work. In all these instances, energy is subject to the laws of thermodynamics. In fact, all processes in a cell, from diffusion to osmosis to the pumping of ions to cell movement, are subject to these laws. There are four laws of thermodynamics, two of which are particularly relevant to biological processes.
6.3.1 The first law of thermodynamics: Energy is conserved.
The first law of thermodynamics is the law of conservation of energy. It states that the universe contains a constant amount of energy. Therefore, energy is neither created nor destroyed. New energy is never formed, and energy is never lost. Instead, energy simply changes from one form to another. For example, kinetic energy can change to potential energy and vice versa, but the total amount of energy always remains the same (Fig. 6.6).
In the simple example of a ball rolling down a set of stairs (see Fig. 6.3), the potential energy stored at the top of the stairs is converted to kinetic energy associated with the movement of the ball and the surrounding air. The total amount of energy in this energy transformation is constant—that is, the difference in potential energy at the top and the bottom of the stairs is equal to the total amount of kinetic energy associated with the movement of the ball and air molecules.
Similarly, when an electron moves from a higher energy level, or shell, to a lower one, it emits light or heat (see Fig. 6.4). The difference in potential energy between the two energy levels is equal to the light or heat energy emitted, consistent with the first law.
6.3.2 The second law of thermodynamics: Disorder tends to increase.
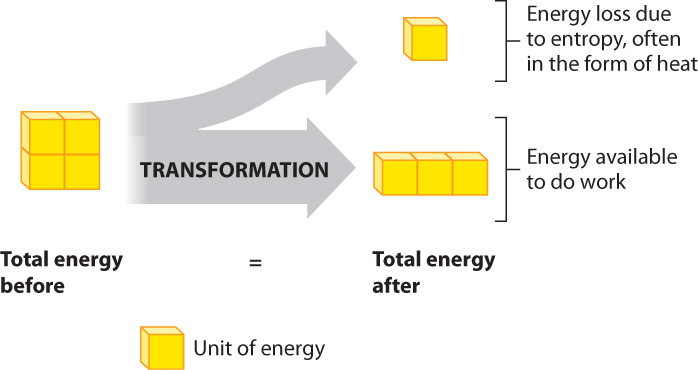
When energy changes form, the total amount of energy remains constant. However, in going from one form of energy to another, the energy available to do work decreases (Fig. 6.7). How can the total energy remain the same, but the energy available to do work decrease? The answer is that some of the energy is available to do work, and some is not available to do work. So, energy transfers are never 100% efficient, since the amount of energy available to do work decreases every time energy changes form.
6-6
The energy that is not available to do work takes the form of an increase in disorder. In other words, there is a universal price to pay in transforming energy from one form to another, which takes the form of an increase in disorder. For example, when kinetic energy is changed into potential energy or vice versa, the amount of disorder always increases. This principle is summarized by the second law of thermodynamics, which states that the transformation of energy is associated with an increase in disorder of the universe. The degree of disorder is called entropy.
In chemical reactions, most of the entropy increase occurs through the transformation of various forms of energy into thermal energy, which we experience as heat. Thermal energy is a form of kinetic energy corresponding to the random motion of molecules, and results in a given temperature. The higher the temperature, the more rapidly the molecules move, and the higher the disorder.
Consider the contraction of a muscle, a form of kinetic energy associated with the shortening of muscle cells. The contraction of muscle is powered by chemical potential energy in fuel molecules. Some of this potential energy is transformed into kinetic energy (movement), and the rest is dissipated as thermal energy (which is why your muscles warm as you exercise). The amount of chemical potential energy is equal to the amount of kinetic energy plus the amount of thermal energy, consistent with the first law of thermodynamics. In addition, because of the entropy “tax,” not all of the chemical energy stored in molecules is converted to kinetic energy, as thermal energy flowing as heat is a necessary by-product of the process.
In living organisms, catabolic reactions result in an increase of entropy as a single ordered biomolecule is broken down into several smaller ones with more freedom to move around, consistent with the second law. Anabolic reactions, by contrast, might seem to decrease entropy because they use individual building blocks to synthesize more ordered biomolecules such as proteins or nucleic acids. Do these anabolic reactions violate the second law of thermodynamics? No, because the second law of thermodynamics always applies to the universe as a whole, not to a chemical reaction in isolation. A local decrease in entropy is always accompanied by an even higher increase in the entropy of the surroundings. The production of heat in a chemical reaction increases entropy because heat is associated with the motion of molecules. The combination of the decrease in entropy associated with the building of a macromolecule and the increase in entropy associated with heat always results in a net increase in entropy.
The key point here is that the maintenance of the high degree of function and organization of a single cell or a multicellular organism requires a constant input of energy, either from the sun or from the energy stored in chemical compounds. This energy allows molecules to be built and work to be carried out, but also leads to greater disorder in the surroundings.