7.1 AN OVERVIEW OF CELLULAR RESPIRATION
In the last chapter, we saw that catabolism describes the set of chemical reactions that break down molecules into smaller units. In the process, these reactions release chemical energy and store it in molecules of ATP. Anabolism, by contrast, is the set of chemical reactions that build molecules from smaller units. Anabolic reactions require an input of energy, usually in the form of ATP.
7-2
Cellular respiration is one of the major sets of catabolic reactions in a cell. During cellular respiration, fuel molecules such as glucose, fatty acids, and proteins are catabolized into smaller units, releasing the energy stored in their chemical bonds to power the work of the cell.
7.1.1 Cellular respiration occurs in four stages.
Cellular respiration is a series of catabolic reactions that convert the energy stored in food molecules, such as glucose, into ATP. It can occur in the presence of oxygen (termed aerobic respiration) or in the absence of oxygen (termed anaerobic respiration). Most organisms are capable of aerobic respiration; some bacteria respire anaerobically (Chapter 26). Here we focus on aerobic respiration. Oxygen is consumed in aerobic respiration, and carbon dioxide and water are produced. The process occurs in four stages (Fig. 7.1).
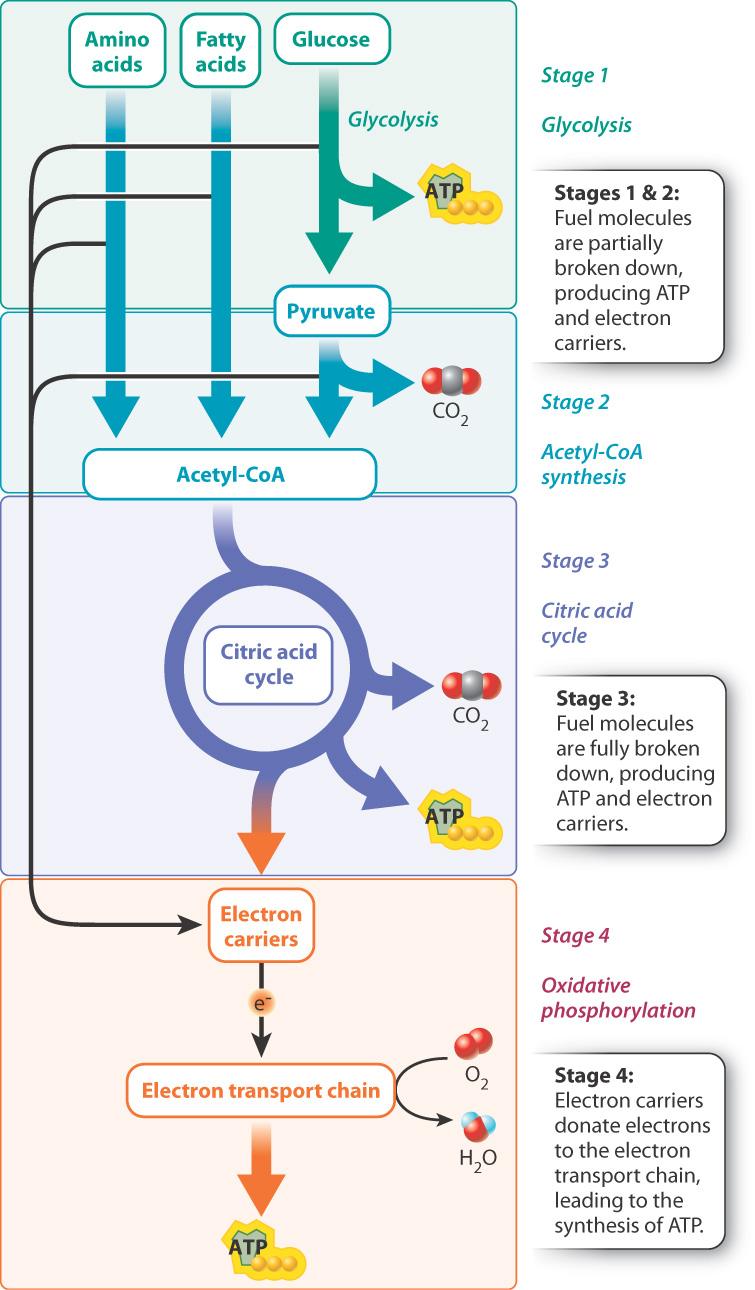
In stage 1, glucose, fatty acids, or amino acids are partially broken down and a modest amount of energy is released. In this chapter, we focus on the breakdown of glucose to make pyruvate, a process known as glycolysis.
In stage 2, pyruvate is converted to another molecule called acetyl-coenzyme A (acetyl-CoA) and carbon dioxide is produced.
Stage 3 is the citric acid cycle. During this stage, acetyl-CoA is broken down and more carbon dioxide is released.
In stages 1–3, chemical energy is transferred to two types of energy-storing molecules: ATP and electron carriers. ATP was discussed in Chapter 6. Electron carriers are molecules that store and transfer energy in the form of “high-energy” or “excited” electrons, discussed more fully below.
Stage 4 is oxidative phosphorylation. In this series of reactions, electron carriers generated in stages 1–3 donate their high-energy electrons to an electron transport chain (also called a respiratory chain). Electron transport chains transfer electrons along a series of membrane-associated proteins to a final electron acceptor and harness the energy of the electrons to produce a large amount of ATP. In aerobic respiration, oxygen is the final electron acceptor, so oxygen is consumed and water is produced in the process.
In eukaryotes, glycolysis takes place in the cytoplasm, and the citric acid cycle and oxidative phosphorylation take place in mitochondria. The electron transport chain is made up of proteins associated with the inner mitochondrial membrane (Chapter 5). In some bacteria, these reactions take place in the cytoplasm, and the electron transport chain is located in the plasma membrane. It is thought that mitochondria were once free-living bacteria but are now unable to live outside their host eukaryotic cell. This idea, known as the endosymbiotic theory, is discussed more fully in Chapter 27.
Before turning to the details of the four stages of cellular respiration, we take a moment to consider how energy is stored and used in cells. In particular, we focus on why carbohydrates and lipids are such good sources of energy.
7.1.2 Cellular respiration involves a series of redox reactions.
You know from everyday experience that carbohydrates and lipids are good sources of energy. The key to understanding how energy is stored in these molecules is to understand oxidation–reduction reactions (“redox reactions” for short). In biological systems, oxidation–reduction reactions are often used to store or release chemical energy.
7-3
Oxidation is the loss of electrons, and reduction is the gain of electrons. The loss and gain of electrons always occur together in a single oxidation–reduction reaction: Electrons are transferred from one molecule to another so that one molecule loses electrons and one molecule gains those electrons.
A familiar example of an oxidation–reduction reaction is the reaction of iron and oxygen to make iron oxide—that is, rust. The overall reaction can be written as follows:
We can also write this reaction as two half-reactions in order to follow the electrons:
As you can see from these half-reactions, iron loses electrons and is therefore oxidized, while oxygen gains electrons and is reduced.
The electrons lost by iron are gained by oxygen, so the original reaction can be written as follows:
In many biological systems, electrons are not completely transferred between molecules. Instead, there is a change in electron density around an atom. In this case, oxidation is a decrease in electron density and reduction is an increase in electron density. Another way to think about these reactions is that electrons are partially lost or gained. The breakdown of the sugar glucose provides an example. The overall reaction can be written as follows:
This reaction is an example of an oxidation–reduction reaction in which glucose is oxidized to carbon dioxide and at the same time oxygen is reduced to water:
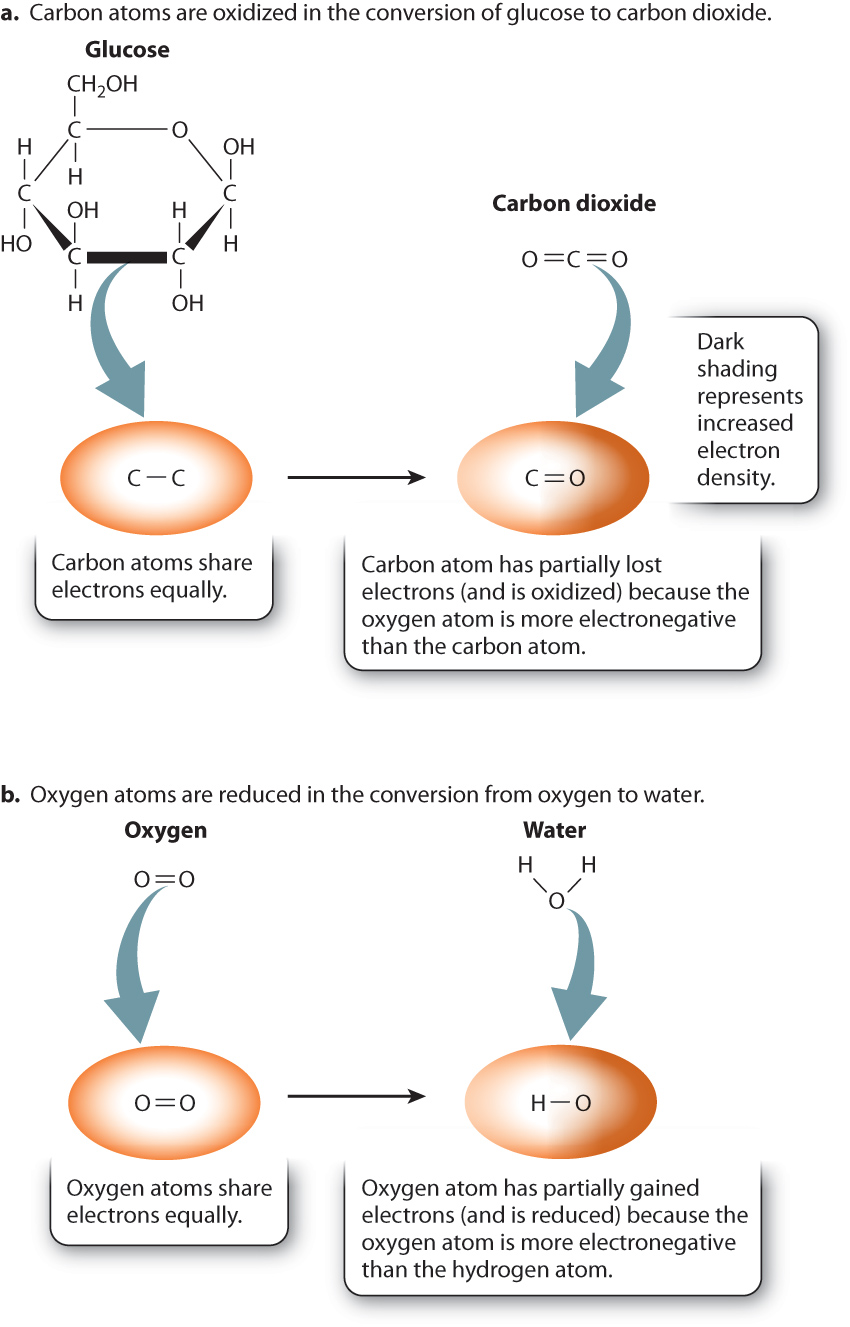
To understand why this is an oxidation–reduction reaction, consider where the electrons are in the reactants and products. Let’s first compare glucose and carbon dioxide. The carbon atoms of glucose are bound to other carbon atoms, hydrogen atoms, and oxygen atoms. In the case of C—C and C—H covalent bonds, electrons are shared about equally between the two atoms (Fig. 7.2). By contrast, in carbon dioxide, they are not shared equally. The oxygen atom is more electronegative than the carbon atom, so the electrons that are shared between carbon and oxygen spend more time near the oxygen atom (Fig. 7.2a). As a result, in the preceding reaction, carbon has partially lost electrons to oxygen. We say that the carbon atoms in glucose are oxidized, or simply that glucose is oxidized.
7-4
Let’s consider the reduction reaction by comparing oxygen gas and water (Fig. 7.2b). In oxygen gas, electrons are shared equally between two oxygen atoms. In water, the electrons that are shared between hydrogen and oxygen spend more time near oxygen because oxygen is more electronegative than hydrogen. As a result, in the preceding reaction, the electron density around the oxygen atom has increased, so oxygen has partially gained electrons and is reduced.
Because oxygen gains electrons, it is called an electron acceptor. It also oxidizes glucose, so it can be referred to as an oxidizing agent. Glucose, conversely, is an electron donor and therefore a reducing agent.
Oxidation and reduction reactions are defined by the loss or gain of electrons. But note that in the oxidation of glucose, electrons always travel with hydrogen atoms. Therefore, the movement of hydrogen atoms can be used as an aid to recognize which molecules become oxidized and which ones become reduced. For example, we can see that the carbon atoms of glucose lose hydrogen atoms in the conversion to carbon dioxide; therefore, glucose loses electrons and is oxidized. In the same reaction, oxygen gains hydrogen atoms in becoming water; therefore, it gains electrons and is reduced. Using hydrogen atoms as a marker for electrons is often a convenient way to follow redox reactions that occur in biological systems. However, the movement of electrons is key, as they are the entities that carry and transfer energy in redox reactions.
7.1.3 Chemical energy is stored in reduced molecules such as carbohydrates and lipids.
We can now address the question of why reduced molecules, such as carbohydrates and lipids, have so much energy. The answer has to do with how the atoms of these molecules share electrons.
The shared electrons of C—C and C—H bonds characteristic of carbohydrates and lipids have high potential energy. The high potential energy results from the fact that the electrons are, on average, far from the nucleus of the atoms. As discussed in Chapter 6, the farther an electron is from the nucleus, the more potential energy it has. By contrast, when electrons are close to the nucleus of an atom, as in the case of carbon dioxide and water, they have lower potential energy. So the oxidation of glucose during cellular respiration results in a large release of energy because the potential energy of the reactants is higher than that of the products.
The reaction for the oxidation of glucose helps us focus on the starting reactants, final products, and release of energy. However, it misses the many intermediate steps that take place as the cell oxidizes glucose. Tossing a match into the gas tank of a car would release a tremendous amount of energy in the form of an explosion, but this energy would not be used productively. Similarly, if all the energy stored in glucose were released at once, most of it would be released as heat and the cell would not be able to harness it to do work. The energy in organic molecules is released gradually in a series of chemical reactions (Fig. 7.3). By oxidizing glucose slowly and in a controlled manner, the chemical energy stored in glucose can be harnessed in the chemical bonds of other molecules, such as ATP and electron carriers. ATP carries energy in its chemical bonds (Chapter 6). Electron carriers contain chemical energy in the form of high-energy electrons, which can be harnessed to produce ATP.
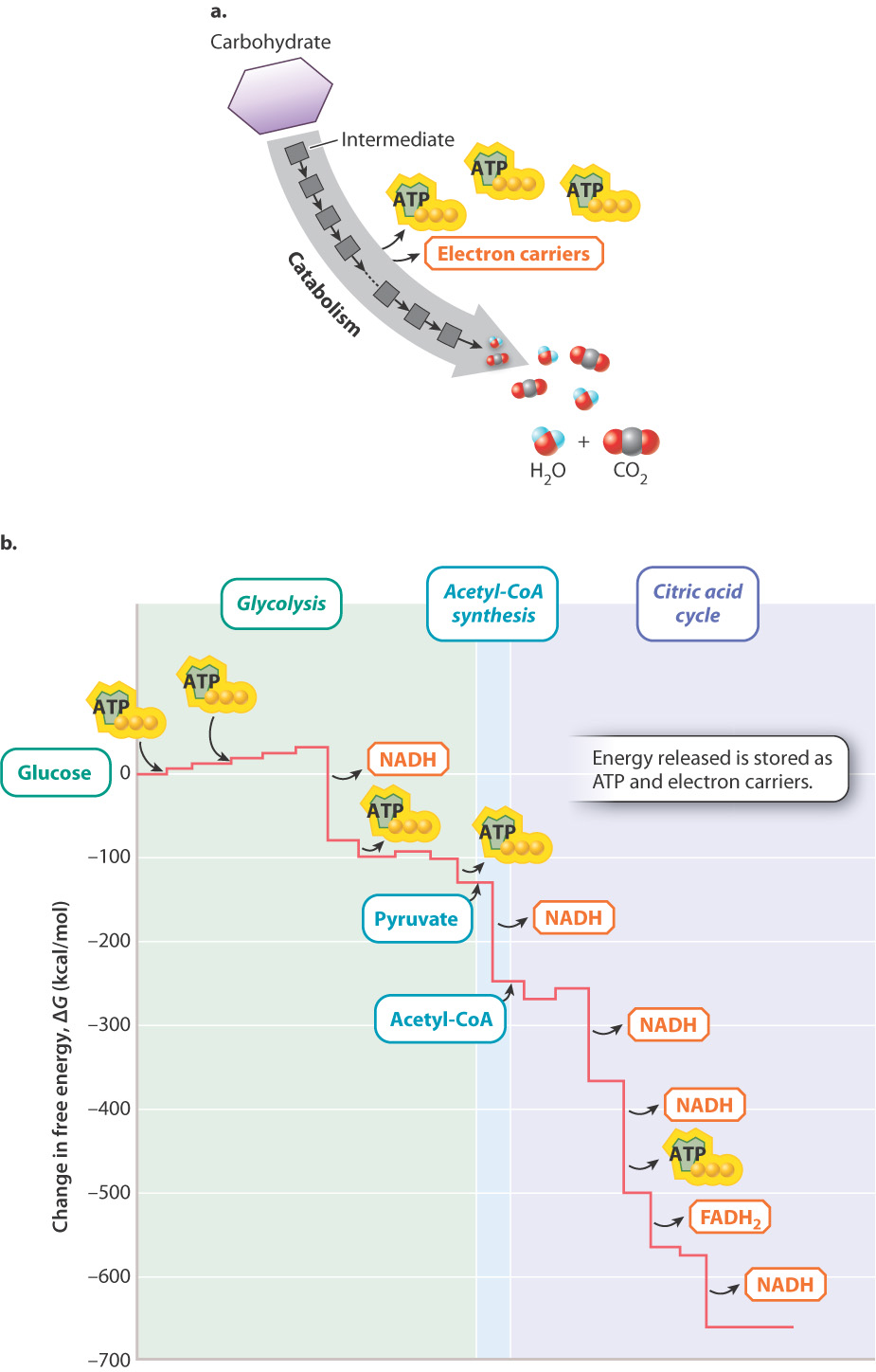
7.1.4 Electron carriers transport high-energy electrons.
As we just saw, many of the chemical reactions involved in cellular respiration are redox reactions that store energy in the form of electron carriers. Two important electron carriers in cells are the molecules nicotinamide adenine dinucleotide (NAD+/NADH) and flavin adenine dinucleotide (FADH/FADH2). These electron carriers exist in two forms—an oxidized form (NAD+ and FAD) and a reduced form (NADH and FADH2). The oxidized form accepts electrons in many of the reactions of cellular respiration and becomes reduced. The reduced form has high potential energy. The electrons it gains in redox reactions are then donated to the electron transport chain. The electron transport chain transfers these electrons to oxygen, the final electron acceptor, releasing energy used to synthesize ATP.
Electron transport chains are used in respiration to extract energy from fuel molecules such as glucose, as well as in photosynthesis to extract energy from sunlight (Chapter 8).
7.1.5 ATP is generated by substrate-level phosphorylation and oxidative phosphorylation.
The chemical energy stored in a molecule of glucose is used to produce ATP in two different ways during cellular respiration. In some reactions, a phosphorylated organic molecule directly transfers a phosphate group to ADP. The free energy difference (ΔG) of hydrolysis of these phosphorylated molecules is more negative than that of ATP hydrolysis and so can drive the synthesis of ATP by energetic coupling (Chapter 6). This way of generating ATP is called substrate-level phosphorylation because a phosphate group is transferred to ADP from an organic molecule, which acts as a phosphate donor or substrate. Substrate-level phosphorylation produces only a small amount of the total ATP generated in the process of cellular respiration.
7-5
Most of the ATP generated in cellular respiration is produced in an entirely different manner— by oxidative phosphorylation. ATP is generated indirectly in the reactions of oxidative phosphorylation, through the reduction of electron carriers, the transfer of high-energy electrons from electron carriers to the electron transport chain, and the subsequent synthesis of ATP from ADP and inorganic phosphate (Pi).