7.4 THE CITRIC ACID CYCLE
The citric acid cycle is the step in cellular respiration in which fuel molecules are completely oxidized. It is also called the Krebs cycle and the tricarboxylic acid (TCA) cycle. During the citric acid cycle, the chemical energy in the bonds of acetyl-CoA is transferred to ATP by substrate-level phosphorylation and to the electron carriers NADH and FADH2. In this way, the citric acid cycle supplies high-energy electrons to the electron transport chain, leading to the production of much more energy in the form of ATP than is obtained by glycolysis alone.
7.4.1 The citric acid cycle produces ATP and electron carriers.
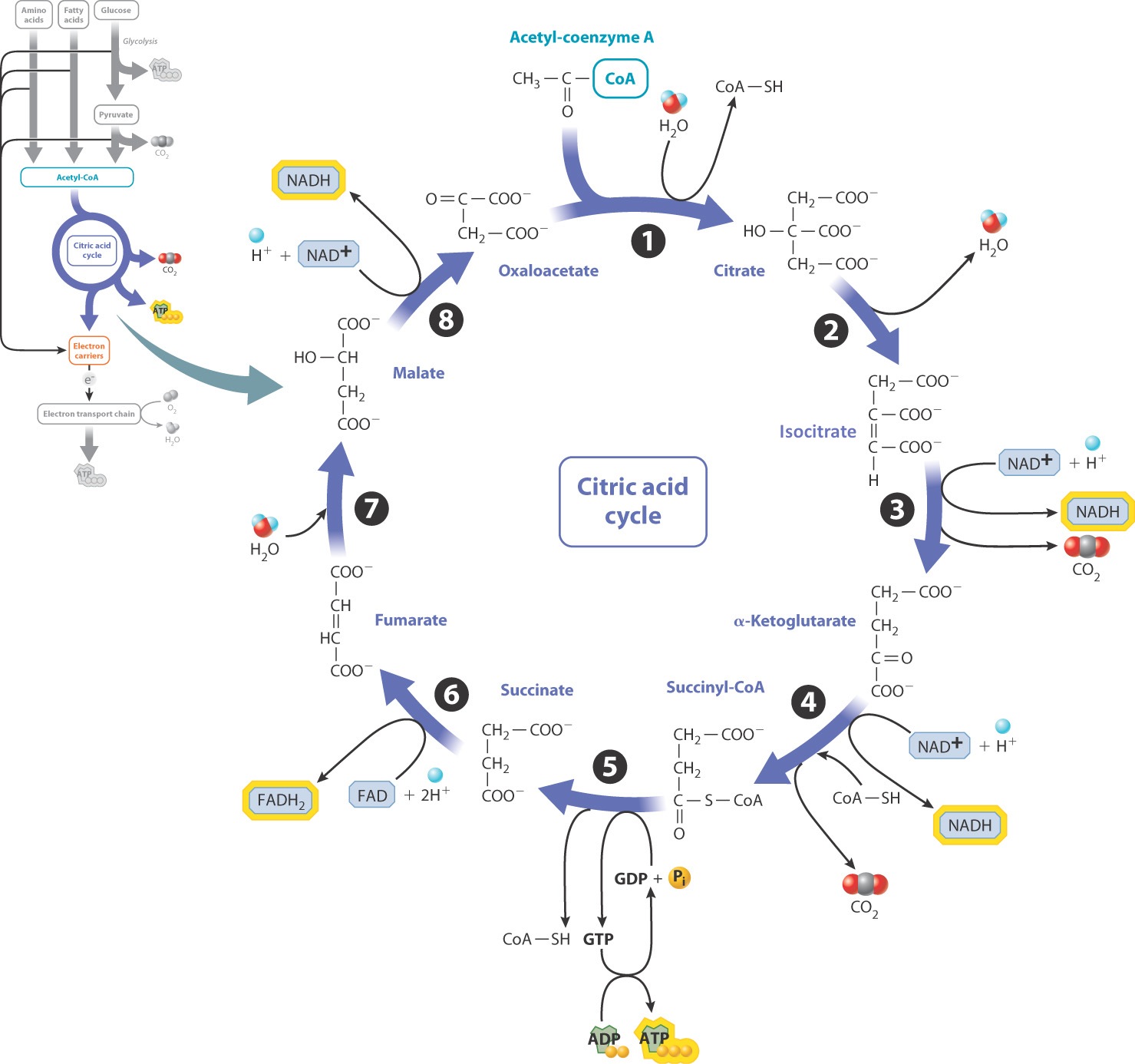
Like the synthesis of acetyl-CoA, the citric acid cycle takes place in the mitochondrial matrix. It is composed of eight reactions and is called a cycle because the starting molecule, oxaloacetate, is regenerated at the end (Fig. 7.7). In the first reaction, a 4-carbon molecule of oxaloacetate reacts with the 2-carbon acetyl group of acetyl-CoA to form a 6-carbon molecule of citrate. The last reaction of the cycle regenerates a molecule of oxaloacetate, allowing the cycle to continue.
7-9
The citric acid cycle results in the complete oxidation of the acetyl group of acetyl-CoA. Since the first reaction creates a molecule with six carbons and the last reaction regenerates a 4-carbon molecule, two carbons are eliminated during the cycle. These carbons are released as carbon dioxide in reactions 3 and 4. Along with the release of carbon dioxide from pyruvate in its conversion to acetyl-CoA, these reactions are the sources of the CO2 released during cellular respiration and therefore the sources of the CO2 that we exhale when we breathe.
The oxidation of carbon atoms to produce CO2 in reactions 3 and 4 is coupled with the reduction of the electron carrier NAD+ to NADH (Fig. 7.7). In this way, energy released in the oxidation reactions is transferred to NADH. Additional electron carriers are produced in reactions 6 and 8, which are also redox reactions. In fact, the citric acid cycle produces a large quantity of electron carriers: three molecules of NADH and one molecule of FADH2 per turn of the cycle. These electron carriers donate high-energy electrons in the next stage of cellular respiration to power the synthesis of ATP.
7-10
Reaction 5 is a substrate-level phosphorylation reaction that generates a molecule of GTP (Fig. 7.7). GTP can transfer its terminal phosphate to a molecule of ADP to form ATP. Reaction 5 is the only substrate-level phosphorylation in the citric acid cycle.
Overall, two molecules of acetyl-CoA produced from a single molecule of glucose yield two molecules of ATP, six molecules of NADH, and two molecules of FADH2 in the citric acid cycle (Table 7.1).
Question Quick Check 3
FzBcwoz3oenyfFK2WJ/UVG4TWGPDc3gGQUL+Vqa9g0gm7ANqSCKYQ/+nTKYPnJ1t76oY+qTeJPg+m3jAMmreK5/C/ha8ENuUnGGB+iDZZARaEN8Awp6VWuSrssyF2ihmts57iFfw2S+TfVbAIaX6GhJAhtknRbczDJ79vC6TqGWn48bGb68+3+P687RHYiS/9DJNbsoCet03rI8yxX2XShGC8OfnJBmqPtww/A==7.4.2 What were the earliest energy-harnessing reactions?
Case 1 The First Cell: Life's Origins
Some bacteria run the citric acid cycle in reverse, incorporating carbon dioxide into organic molecules instead of liberating it. Running the citric acid cycle in reverse requires energy, which is supplied by sunlight (Chapter 8) or chemical reactions (Chapter 26).
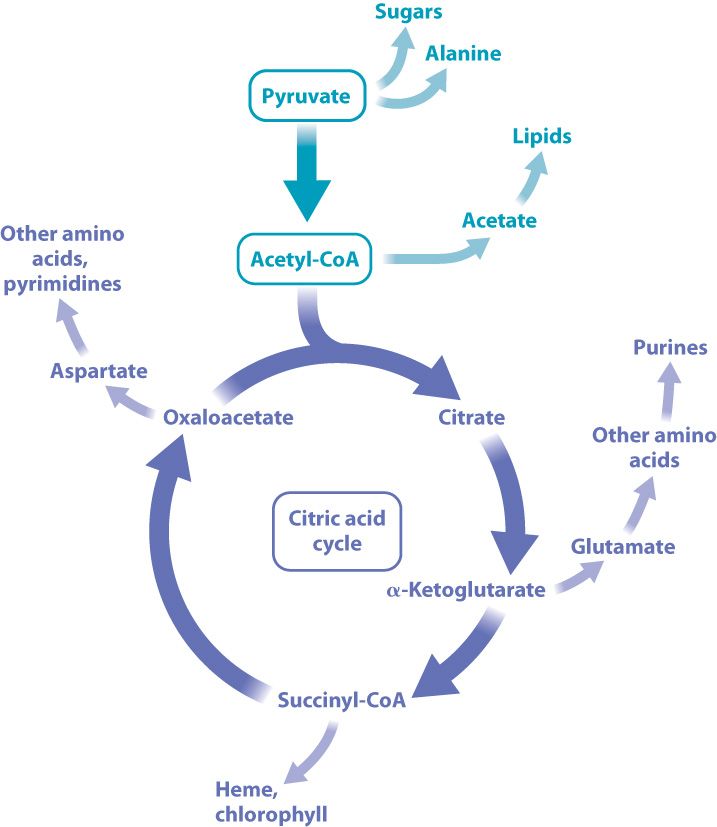
Why would an organism run the citric acid cycle in reverse? The answer is that the intermediates generated step by step as the cycle turns provide the building blocks for synthesizing the cell’s key biomolecules. This is true whether the cycle is run in the reverse or forward direction (Fig. 7.8). Pyruvate, for example, is the starting point for the synthesis of sugars and the amino acid alanine; acetate is the starting point for the synthesis of the cell’s lipids; oxaloacetate is modified to form different amino acids and pyrimidine bases; and α- (alpha-) ketoglutarate is modified to form other amino acids. For organisms that run the cycle in the forward direction, the citric acid cycle is used to generate both energy-storing molecules (ATP and electron carriers) and intermediates in the synthesis of other molecules. For organisms that run the cycle in reverse, it is used to generate intermediates in the synthesis of other molecules and also to reduce carbon dioxide and incorporate carbon into organic molecules.
The centrality of the citric acid cycle to both the synthesis of biomolecules and to meeting the energy requirements of cells suggests to some biologists that this cycle evolved early, appearing in some of the first cells to feature metabolism. This early appearance of the citric acid cycle, in turn, implies that the great variety of biosynthetic and energy-yielding pathways found in modern cells evolved through the extension and modification of this deeply rooted cycle. As always in evolution, new cellular capabilities arose by the modification of preexisting capabilities. Complex networks of highly specific pathways evolved from a simpler, more general set of reactions.