7.6 ANAEROBIC METABOLISM AND THE EVOLUTION OF CELLULAR RESPIRATION
Up to this point, we have followed a single metabolic path: the breakdown of glucose in the presence of oxygen to produce carbon dioxide and water. However, metabolic pathways more often resemble intersecting roads rather than a single, linear path. We saw this earlier in the discussion of the citric acid cycle, where intermediates in the cycle often feed into other metabolic pathways.
One of the major forks in the metabolic road occurs at pyruvate, the end product of glycolysis (section 10.2). When oxygen is present, it is converted to acetyl-CoA, which then enters the citric acid cycle, resulting in the production of ATP and electron carriers to fuel the electron transport chain, as we saw. When oxygen is not present, however, pyruvate is metabolized along a number of different pathways. These pathways occur in many living organisms today and played an important role in the early evolution of life on Earth.
7-15
7.6.1 Fermentation extracts energy from glucose in the absence of oxygen.
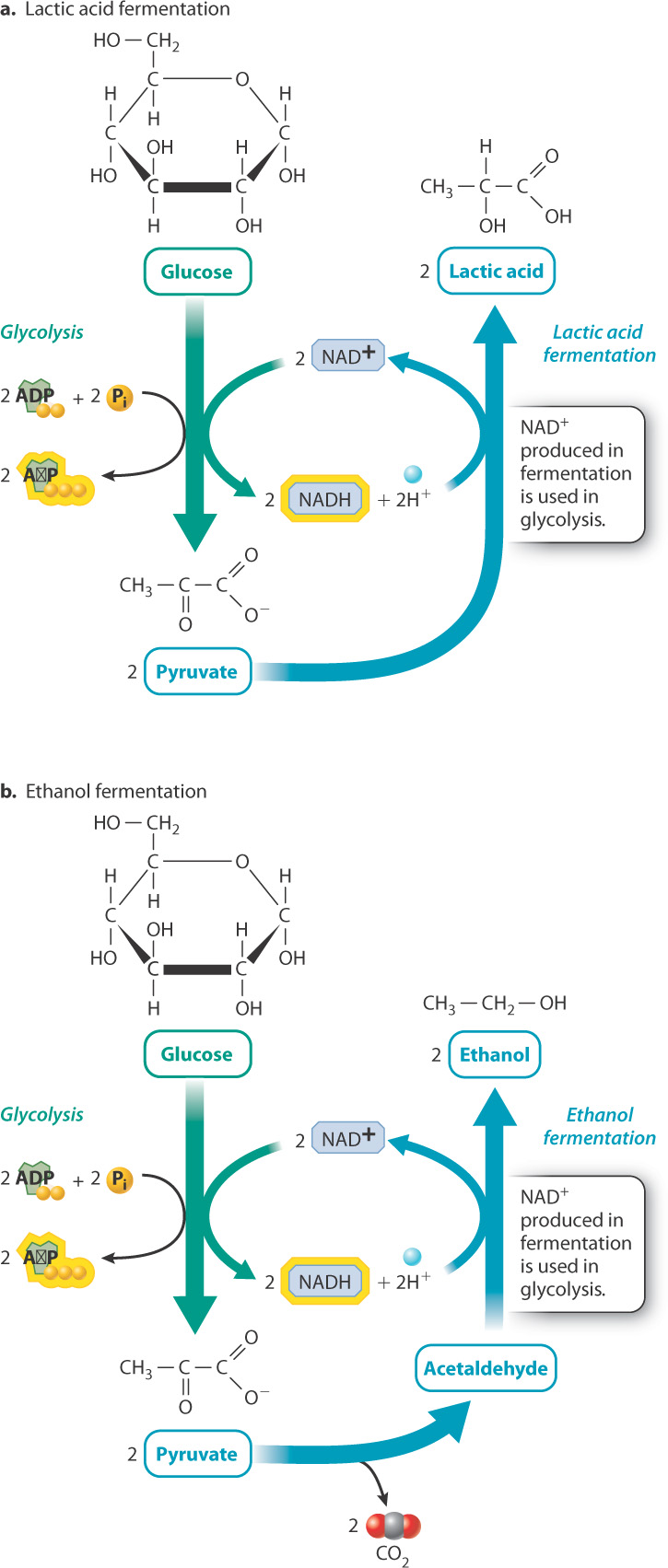
Pyruvate, the end product of glycolysis, is a molecule shared by many metabolic pathways and it therefore has many possible fates in the cell. In the presence of oxygen, most of the pyruvate is used to synthesize acetyl-CoA, which in turn fuels the citric acid cycle. In the absence of oxygen, pyruvate can be broken down by fermentation, which does not rely on oxygen or a similar outside electron acceptor. Fermentation is accomplished through a wide variety of metabolic pathways that extract energy from fuel molecules such as glucose. Fermentation pathways are important for anaerobic organisms that live without oxygen, as well as some organisms such as yeast that favor fermentation over oxidative phosphorylation even in the presence of oxygen. It is also sometimes used in aerobic organisms when oxygen cannot be delivered fast enough to meet the cell’s metabolic needs, as in exercising muscle.
Recall that during glycolysis, glucose is oxidized to form pyruvate, and NAD+ is reduced to form NADH. For glycolysis to continue, NADH must be oxidized to NAD+. The regeneration of NAD+ is important because without it glycolysis would grind to a halt. In the presence of oxygen, NAD+ is regenerated when NADH donates its electrons to the electron transport chain. In the absence of oxygen during fermentation, NADH is oxidized to NAD+ when pyruvate or a derivative of pyruvate is reduced.
There are many fermentation pathways, especially in bacteria. Two of the major ones are lactic acid fermentation and ethanol fermentation (Fig. 7.13). Lactic acid fermentation occurs in animals and bacteria. During lactic acid fermentation, electrons from NADH are transferred to pyruvate to produce lactic acid and NAD+ (Fig. 7.13a). The overall chemical reaction is written as follows:
Glucose + 2 ADP + 2 P1 → 2 lactic acid + 2 ATP + 2H2O
Ethanol fermentation occurs in plants and fungi. During ethanol fermentation, pyruvate releases carbon dioxide to form acetaldehyde, and electrons from NADH are transferred to acetaldehyde to produce ethanol and NAD+ (Fig. 7.13b). The overall chemical reaction is written as follows:
Glucose + 2 ADP + 2 P1 → 2 ethanol + 2CO2 + 2 ATP + 2H2O
In both fermentation pathways, NADH is oxidized to NAD+. However, NADH and NAD+ do not appear in the overall chemical equations because there is no net production or loss of either molecule. NAD+ molecules that are reduced during glycolysis are oxidized when lactic acid or ethanol is formed.
7-16
The breakdown of a molecule of glucose by fermentation yields only two molecules of ATP. The energetic gain is relatively small compared to the total yield of aerobic respiration because the end products, lactic acid and ethanol, still contain a large amount of chemical energy in their bonds and are not fully oxidized. The modest yield explains why organisms that produce ATP by fermentation must consume a large quantity of fuel molecules to power the cell.
Question Quick Check 6
P/HDApCHvd6J5XVv3wwc6Y8LpKDJaANdcXtdSnXk1mqnVJn8h8bWUW1spHJpWO0GSdn95w8SgJ9nceDBL63E2yhpYRDtdHjbBpO1xXbv3VLrwtciZVsGbtg5saXb2UaA7ikTj2Pu1kgjPtk0GMwS8Cyavck37wStn+UzQA==7.6.2 How did early cells meet their energy requirements?
Case 1 The First Cell: Life's Origins
The four stages of cellular respiration lead to the full oxidation of glucose, resulting in the release of a large amount of energy stored in its chemical bonds. The first stage, glycolysis, results in only the partial oxidation of glucose, so just some of the energy held in its chemical bonds is released. Nearly all organisms are capable of partially breaking down glucose, suggesting that glycolysis evolved very early in the history of life.
Life first evolved about 4 billion years ago in the absence of atmospheric oxygen. The earliest organisms probably used one of the fermentation pathways to generate the ATP necessary to power cellular processes because fermentation does not require atmospheric oxygen. This process occurs in the cytoplasm and does not require proteins embedded in specialized membranes.
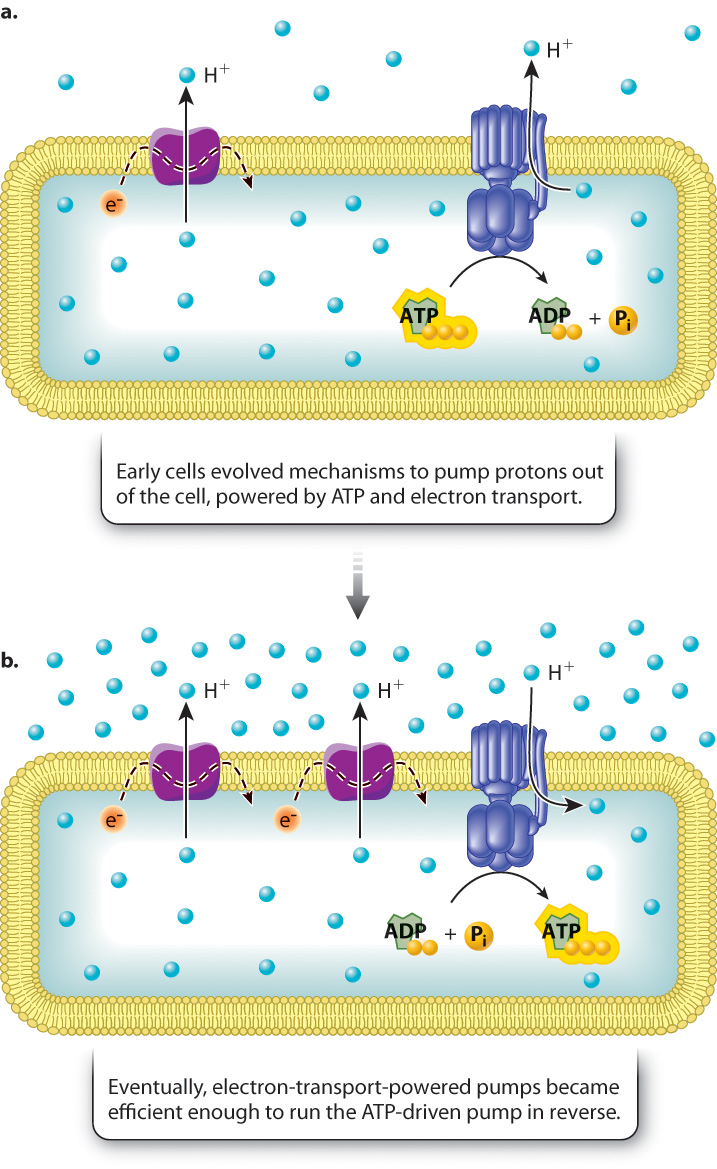
As we have seen, cellular respiration involves an electron transport chain, composed of proteins embedded in a membrane and capable of transferring high-energy electrons from one protein to the next and pumping protons. The resulting proton gradient powers the synthesis of ATP. Like fermentation, cellular respiration can occur in the absence of oxygen, but in that case molecules other than oxygen, such as sulfate and nitrate, are the final electron acceptor (Chapter 26). This form of respiration is known as anaerobic respiration and occurs in some present-day bacteria. The electron transport chain in these bacteria is located in the plasma membrane, not in an internal membrane.
How might such a system have evolved? An intriguing possibility is that early prokaryotes evolved pumps to drive protons out of the cell in response to an increasingly acidic environment (Fig. 7.14). Some pumps might have used the energy of ATP to pump protons, while others used electron transport proteins to pump protons (Fig. 7.14a). At some point, proton pumps powered by electron transport might have become efficient enough that the protons could pass back through the ATP-driven pumps, running them in reverse to synthesize ATP (Fig. 7.14b).
Organisms capable of producing oxygen, the cyanobacteria, did not evolve until about 2.5 billion years ago, maybe earlier. The evolution of this new form of life introduced oxygen into Earth’s atmosphere. This dramatic change led to the evolution of new life-forms with new possibilities for extracting energy from fuel molecules such as glucose. Aerobic respiration, in which oxygen serves as the final electron acceptor in the electron transport chain, generates much more energy than does anaerobic respiration or fermentation.
7-17
The evolution of cellular respiration illustrates that evolution often works in a stepwise fashion, building on what is already present. In this case, aerobic respiration picked up where anaerobic respiration left off, making it possible to harness more energy from organic molecules to power the work of the cell.