7.7 METABOLIC INTEGRATION
In this chapter, we have focused on the breakdown of glucose. What happens if there is more glucose than is needed by the cell? As well as glucose, you probably consume diverse carbohydrates, lipids, and proteins. How are these broken down? And how are these various metabolic pathways coordinated so that the intracellular level of ATP is maintained in a narrow range? In this final section, we consider how the cell responds to these challenges.
7.7.1 Excess glucose is stored as glycogen in animals and starch in plants.
Glucose is a readily available form of energy in organisms, but it is not always broken down immediately. Excess glucose can be stored in cells and then mobilized—that is, broken down—when necessary. Glucose can be stored in two major forms: as glycogen in animals and starch in plants (Fig. 7.15). Both these molecules are large branched polymers of glucose.
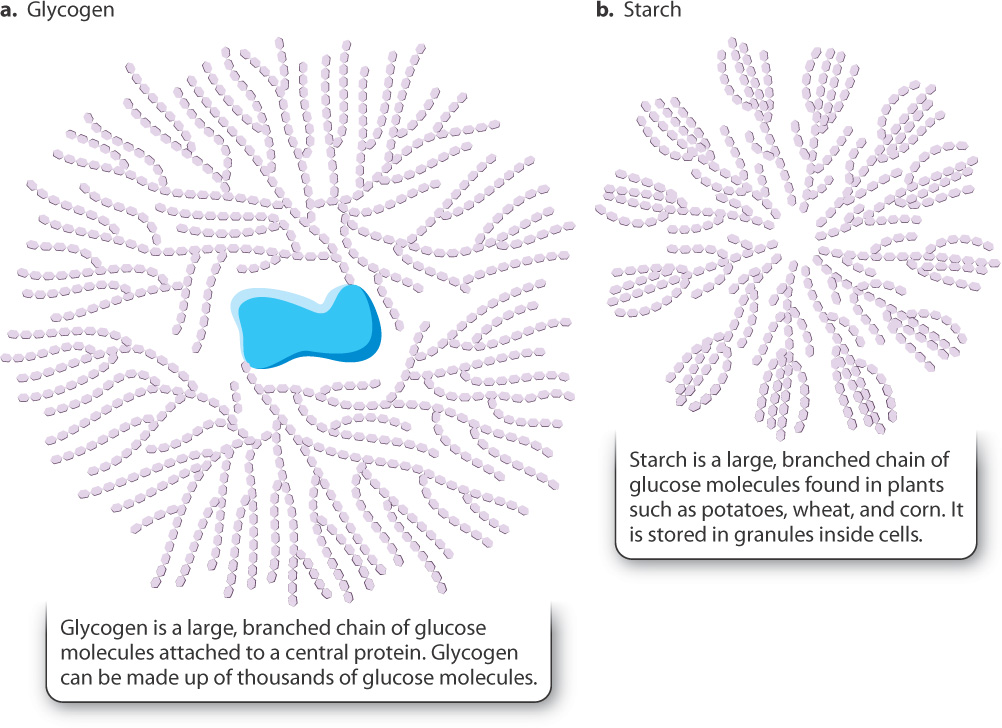
Carbohydrates that are consumed by animals are broken down into simple sugars and circulate in the blood. The level of glucose in the blood is tightly regulated. When the blood glucose level is high, as it is after a meal, glucose molecules that are not consumed by glycolysis are linked together to form glycogen in liver and muscle. Glycogen stored in muscle is used to provide ATP for muscle contraction. By contrast, the liver does not store glycogen primarily for its own use, but is a central glycogen storehouse for the whole body, able to release glucose into the bloodstream when it is needed elsewhere. Glycogen provides a source of glucose 6-phosphate to feed glycolysis when the level of blood glucose is low. Glucose molecules located at the end of glycogen chains can be cleaved one by one, and they are released in the form of glucose 1-phosphate. Glucose 1-phosphate is then converted into glucose 6-phosphate, an intermediate in glycolysis (see Fig. 7.4). One glucose molecule cleaved off a glycogen chain produces three and not two molecules of ATP by glycolysis because the ATP-consuming step 1 of glycolysis is bypassed.
7.7.2 Sugars other than glucose contribute to glycolysis.
The carbohydrates in your diet are digested to produce a variety of sugars (Fig. 7.16). Some of these are disaccharides (maltose, lactose, and sucrose) with two sugar units; others are monosaccharides (fructose, mannose, and galactose) with a single sugar unit. The disaccharides are hydrolyzed into monosaccharides, which are transported into cells.
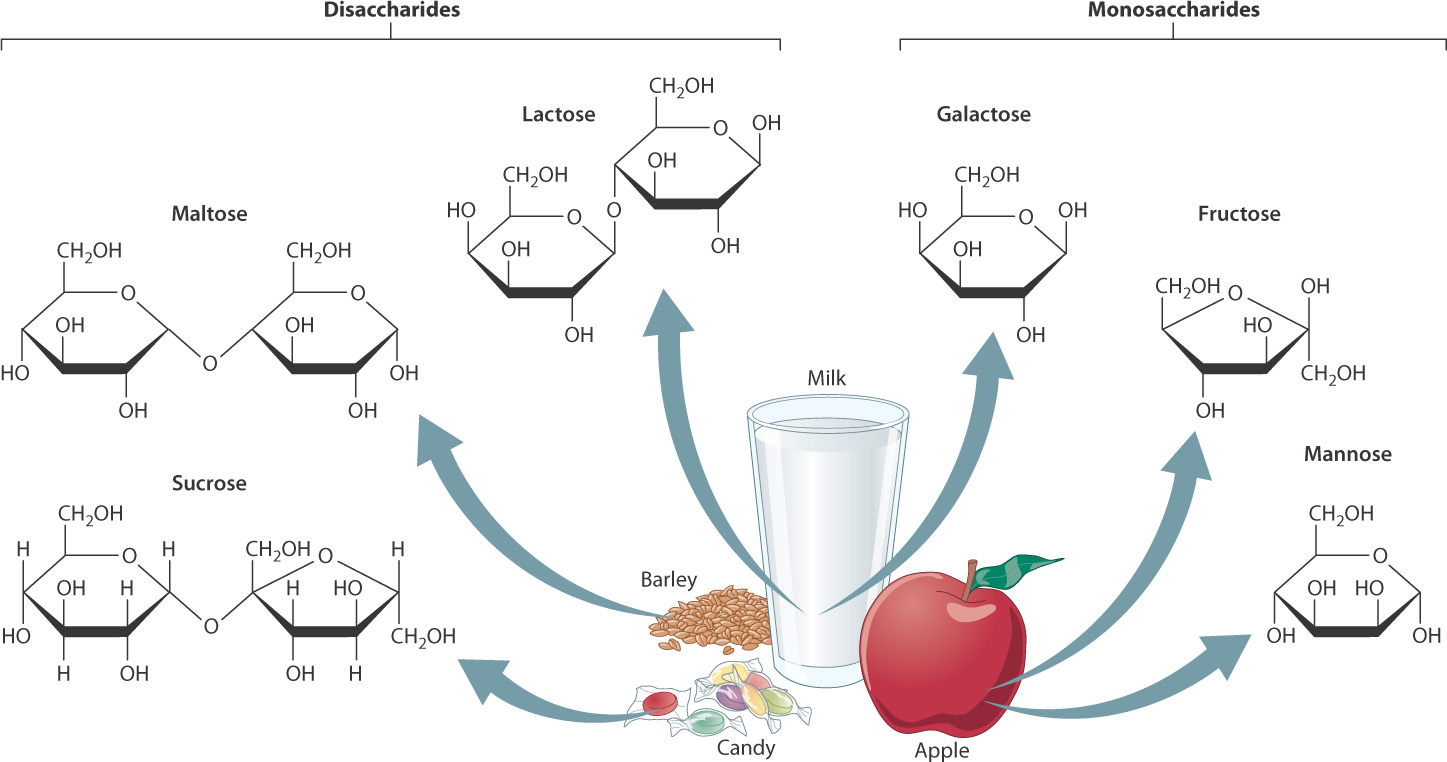
The hydrolysis of some disaccharides produces glucose molecules that directly enter glycolysis. What happens to other monosaccharides? They, too, enter glycolysis, although not as glucose. Instead, they are converted into intermediates of glycolysis that come later in the pathway. For example, fructose is produced by the hydrolysis of sucrose (table sugar) and receives a phosphate group to form either fructose 6-phosphate or fructose 1-phosphate. In the liver, fructose 1-phosphate is cleaved and converted into glyceraldehyde 3-phosphate, which enters glycolysis at reaction 6 (see Fig. 7.4).
7-18
7.7.3 Fatty acids and proteins are useful sources of energy.
In addition to carbohydrates, lipids are also a good source of energy. We know this from common experience. Butter, oils, ice cream, and the like all contain lipids and are high in calories, which are units of energy. We can also infer that lipids are a good source of energy from their chemical structure. Recall from Chapter 2 that a type of fat called triacylglycerol is composed of three fatty acid molecules bound to a glycerol backbone. These fatty acid molecules are rich in carbon–carbon and carbon–hydrogen bonds, which, as we saw earlier, carry chemical energy.
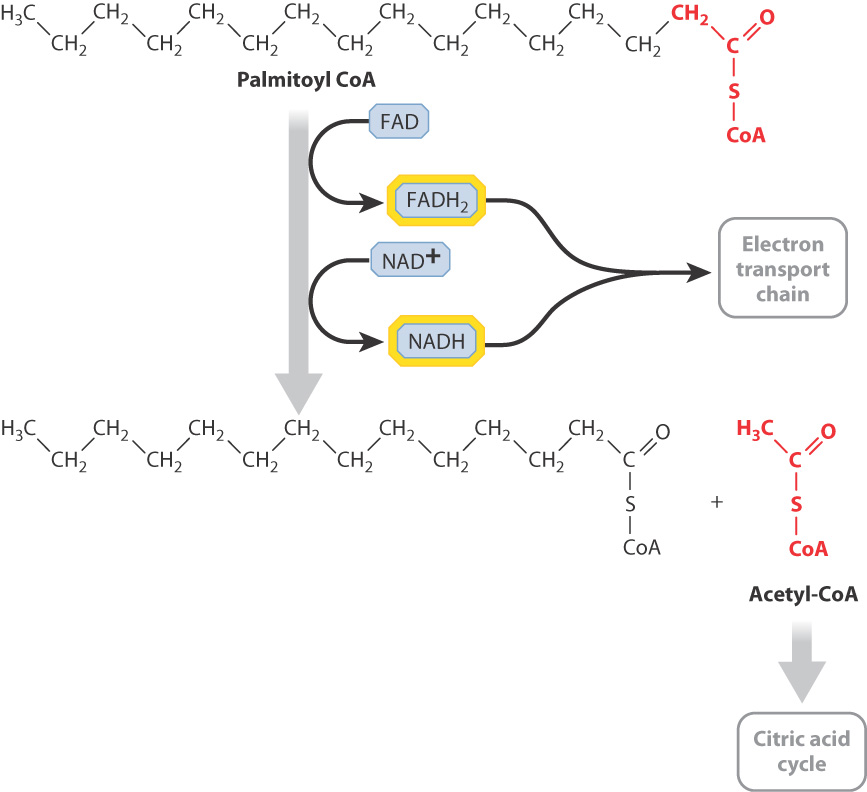
Following a meal, the small intestine very quickly absorbs triacylglycerols, which are then transported by the bloodstream and either consumed or stored in fat (adipose) tissue. Triacylglycerols are broken down inside cells to glycerol and fatty acids. Then, the fatty acids themselves are shortened by a series of reactions that sequentially remove two carbon units from their ends (Fig. 7.17). This process is called β-(beta-) oxidation. It does not produce ATP, but releases a large number of NADH and FADH2 molecules that provide high-energy electrons for the synthesis of ATP by oxidative phosphorylation. In addition, the end product of the reaction is acetyl-CoA, which feeds the citric acid cycle and leads to the production of an even larger quantity of electron carriers.
7-19
The oxidation of fatty acids produces a large amount of ATP. For example, the complete oxidation of a molecule of palmitic acid, a fatty acid containing 16 carbons, yields about 106 molecules of ATP. By contrast, glycolysis yields just 2 molecules of ATP, and the complete oxidation of a glucose molecule produces about 32 molecules of ATP (Table 7.1). Fatty acids therefore are a useful and efficient source of energy, but they cannot be used by all tissues of the body. Notably, the brain and red blood cells depend solely on glucose for energy.
Proteins, like fatty acids, are a source of chemical energy that can be broken down, if necessary, to power the cell. Proteins are typically first broken down to amino acids, some of which can then enter glycolysis and others the citric acid cycle.
7.7.4 The intracellular level of ATP is a key regulator of cellular respiration.
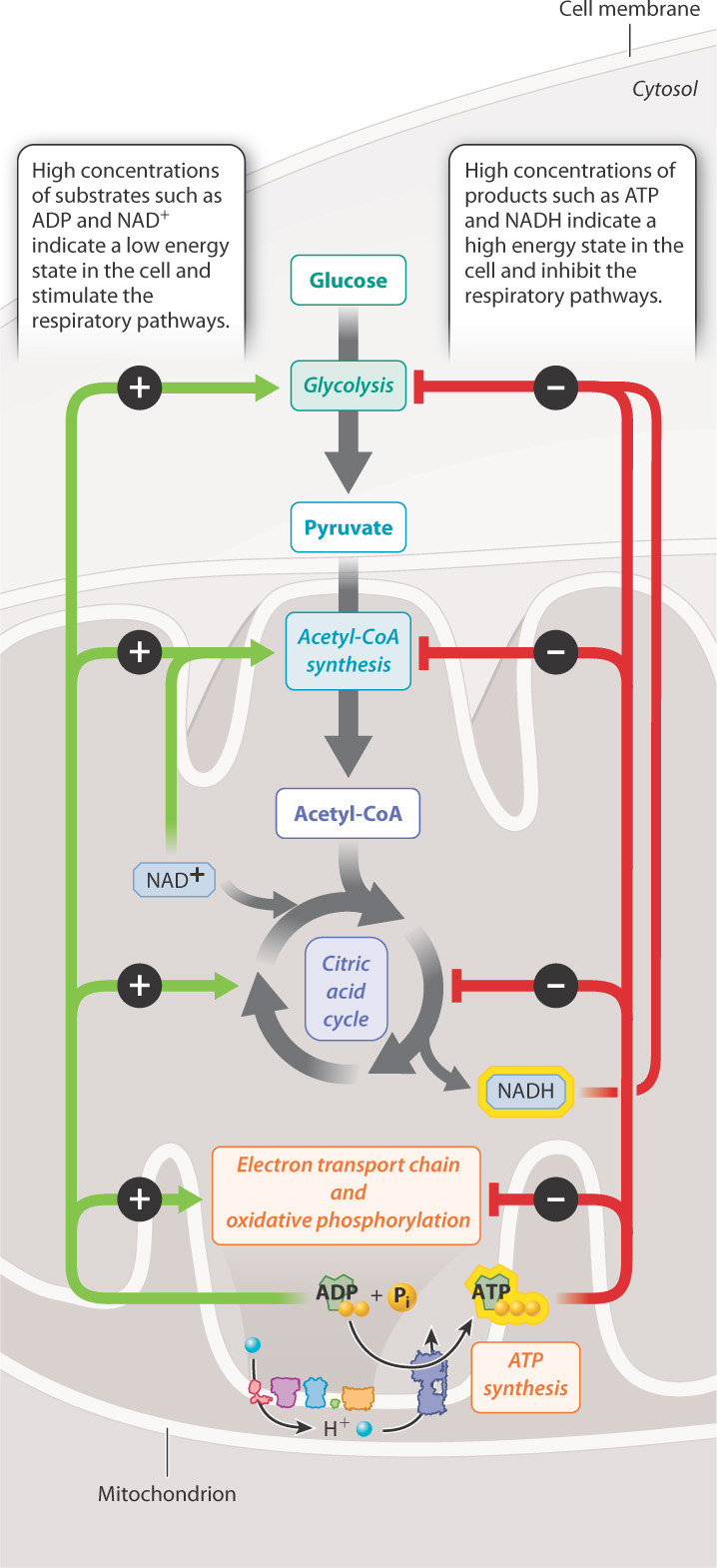
ATP is the key end product of cellular respiration, holding in its bonds energy that can be used for all kinds of cellular processes. ATP is constantly being turned over in a cell, broken down to ADP and Pi to supply the cell’s energy needs, and re-synthesized by fermentation and cellular respiration. The level of ATP inside a cell can therefore be an indicator of how much energy a cell has available. When ATP levels are high, the cell has a high amount of free energy and is poised to carry out cellular processes. In this case, pathways that generate ATP are slowed, or down-regulated. By contrast, when ATP levels are low, the cell activates, or up-regulates, pathways that lead to ATP synthesis. Other intermediates of cellular respiration, such as NADH, have a similar effect in that high NAD1 levels stimulate cellular respiration, whereas high NADH levels inhibit it (Fig. 7.18).
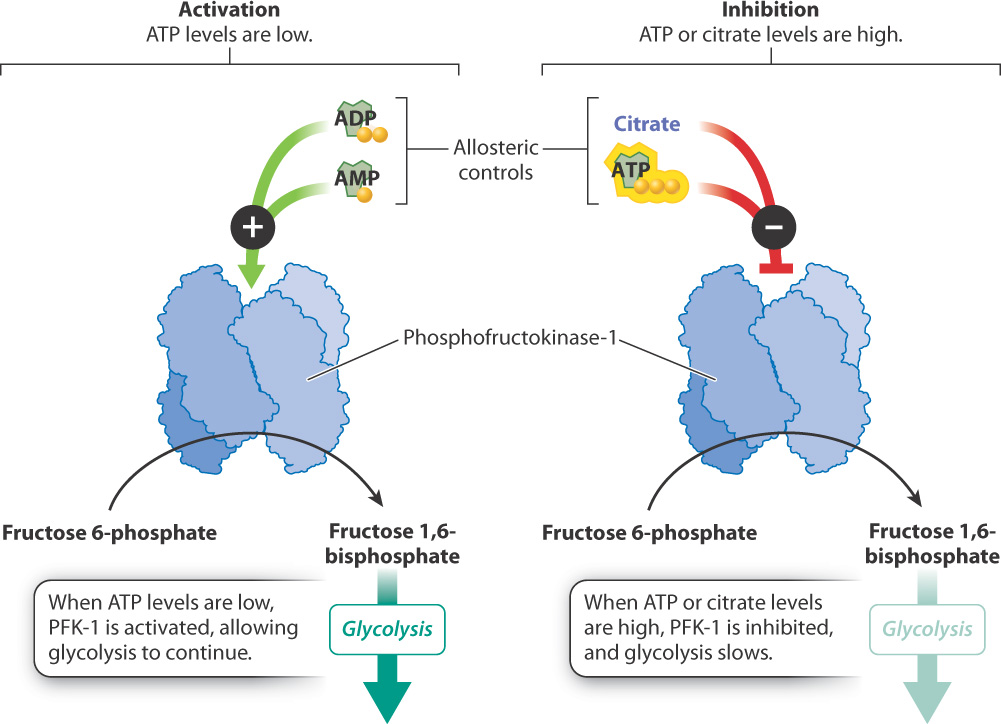
How is this kind of coordinated response of the cell possible? The cell uses several mechanisms, one of which is the regulation of enzymes that control key steps of the pathway. One of these key reactions is reaction 3 of glycolysis—the conversion of fructose 6-phosphate to fructose 1,6-bisphosphate (see Fig. 7.4). This is a key step in glycolysis because it is highly endergonic and irreversible. As a result, it is considered a “committed” step and is subject to tight control. This reaction is catalyzed by the enzyme phosphofructokinase-1 (PFK-1), which can be thought of as a metabolic valve that regulates the rate of glycolysis.
7-20
PFK-1 is an allosteric enzyme with many activators and inhibitors (Fig. 7.19). Recall from Chapter 6 that an allosteric enzyme changes its shape and activity in response to the binding of molecules at a site other than the active site. ADP and AMP are allosteric activators of PFK-1. When ADP and AMP are abundant, one or the other will bind to the enzyme and cause the enzyme’s shape to change. The shape change activates the enzyme, increasing the rate of glycolysis and the synthesis of ATP. When ATP is in abundance, it binds to the same site on the enzyme as ADP and AMP, but in this case binding inhibits the enzyme’s catalytic activity. As a result, glycolysis and the rate of ATP production slow down.
PFK-1 is also regulated by one of its downstream products, citrate, an intermediate in the citric acid cycle (see Fig. 7.7). Citrate acts as an allosteric inhibitor of the enzyme, slowing its activity. High levels of citrate indicate that it is not being consumed and glucose breakdown can be slowed. The role of citrate in controlling glycolysis illustrates the coordinated regulation of glycolysis and the citric acid cycle.
7.7.5 Exercise requires several types of fuel molecules and the coordination of metabolic pathways.
In the last two chapters, we considered what energy is and how it is harnessed by cells. Let’s apply the concepts we discussed to a familiar example: exercise. Exercise such as running, walking, and swimming is a form of kinetic energy, powered by ATP in muscle cells. Where does this ATP come from?
Muscle cells, like all cells, do not contain a lot of ATP, and stored ATP is depleted by exercise in a matter of seconds. As a result, muscle cells rely on fuel molecules to generate ATP. For a short sprint or a burst of activity, muscle can convert stored glycogen to glucose, and then break down glucose anaerobically to pyruvate and lactic acid by lactic acid fermentation. This pathway is rapid, but it does not generate a lot of ATP. In addition, it is limited by the production of lactic acid, which lowers the pH of the blood.
For longer, more sustained exercise, other metabolic pathways come into play. Muscle cells contain many mitochondria, which produce ATP by aerobic respiration. The energy yield of aerobic respiration is much greater than that of fermentation, but the process is slower. This slower production of ATP by aerobic respiration in part explains why runners cannot maintain the pace of a sprint for longer runs.
For even longer exercise, liver glycogen supplements muscle glycogen: The liver releases glucose into the blood that is taken up by muscle cells and oxidized to produce ATP. In addition, fatty acids are released from adipose tissue and taken up by muscle cells, where they are broken down by β-oxidation. β-oxidation yields even more ATP than does the complete oxidation of glucose, but the process is again slower. Storage forms of energy molecules, such as fatty acids and glycogen, contain large reservoirs of energy, but are slower to mobilize. Thus, exercise takes coordination between different cells, tissues, and metabolic pathways to ensure adequate ATP to meet the needs of working muscle.
7-21