8.3 CAPTURING SUNLIGHT INTO CHEMICAL FORMS
To use sunlight to power the Calvin cycle, the cell must be able to convert light energy into both NADPH and ATP. NADPH is the reducing agent used to synthesize carbohydrates from CO2, whereas ATP is required for the regeneration of RuBP. The production of both molecules is the task of the photosynthetic electron transport chain. NADPH is produced by the linear flow of electrons through the photosynthetic electron transport chain from water to NADP+. To produce ATP, the photosynthetic electron transport chain functions as a proton pump, leading to the accumulation of protons in the thylakoid lumen. The resulting proton gradient is used to drive the synthesis of ATP, just as it drives the synthesis of ATP in mitrochondrial respiration (Chapter 7).
8.3.1 Photosystems use light energy to set the photosynthetic electron transport chain in motion.
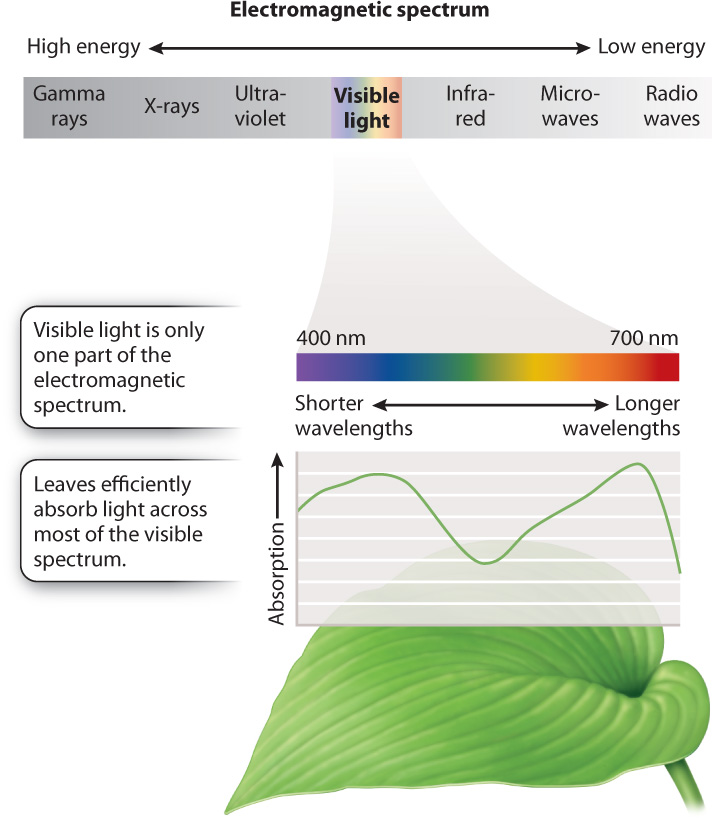
The function of a photosystem is to convert absorbed light energy into the movement of electrons, making it a key element in the photosynthetic electron transport chain. To understand how a photosystem works, we need to know a little about light. Light is a type of electromagnetic radiation, as are radio waves, X-rays, and other forms of radiation. Electromagnetic radiation is energy in the form of waves; the type of electromagnetic energy depends on the wavelength. Visible light is the portion of the electromagnetic spectrum apparent to our eyes, and it includes the range of wavelengths used in photosynthesis. The wavelengths of visible light are from 400 nm to 700 nm.
Pigments are molecules that absorb some wavelengths of visible light (Fig. 8.9). Pigments look colored because they reflect light enriched in the wavelengths that they do not absorb. Physically, a photosystem is a complex of proteins and pigments that is embedded in the thylakoid membrane. Chlorophyll is the major photosynthetic pigment; it appears green because it is poor at absorbing green wavelengths. The chlorophyll molecule consists of a large, light-absorbing “head” containing a magnesium atom at its center and a long hydrocarbon “tail” that allows the pigment to be anchored in the lipid membrane.
The thylakoid membrane also contains other pigments, most notably the orange-yellow carotenoids. Carotenoids are able to absorb light from regions of the visible spectrum that are poorly absorbed by chlorophyll. Thus, the presence of these accessory pigments allows photosynthetic cells to absorb a broader range of visible light than would be possible with just chlorophyll alone. As we will see in section 8.4, carotenoids play an important role in protecting the photosynthetic electron transport chain from damage.
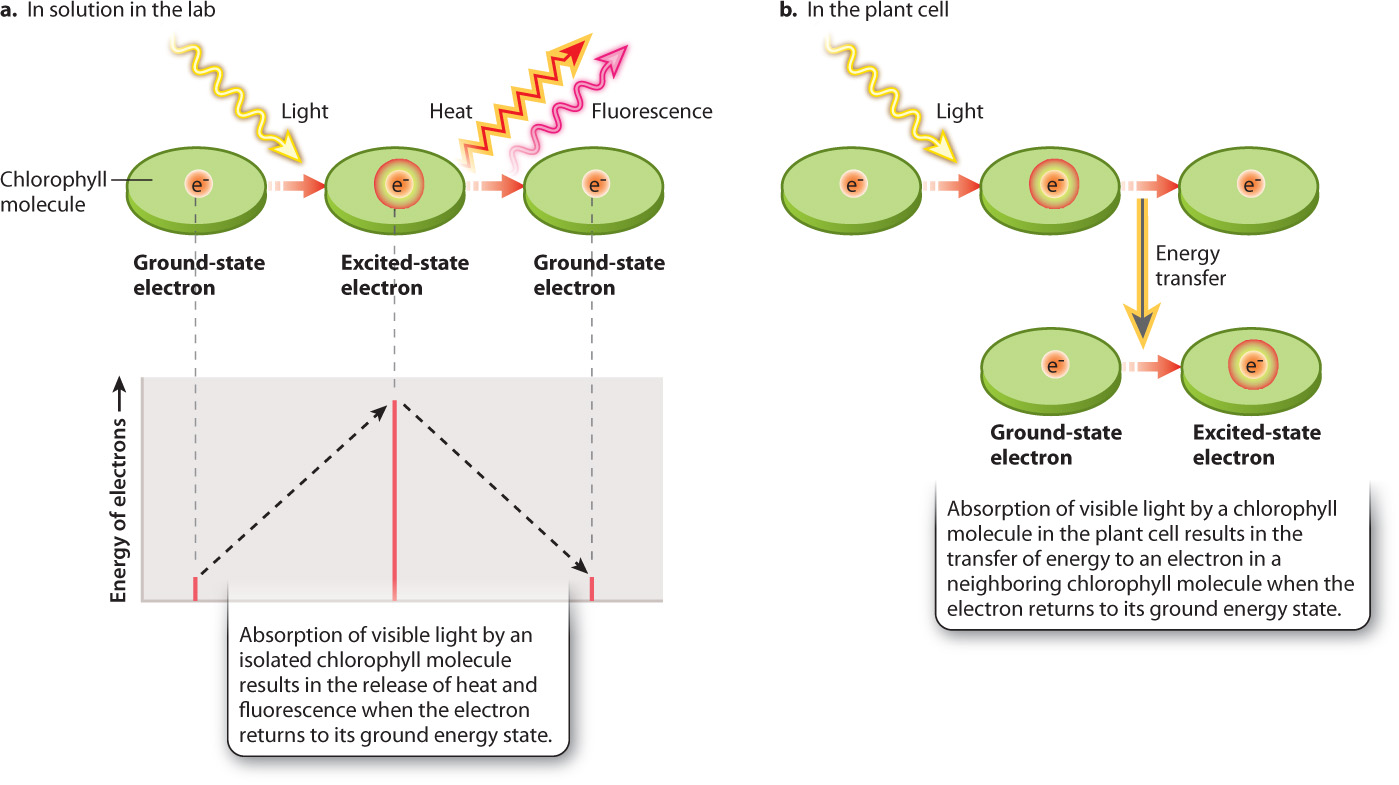
Absorption of visible light by a chlorophyll molecule results in one of its electrons being elevated to a higher energy state (Fig. 8.10). For chlorophyll molecules that have been isolated in the laboratory, this absorbed light energy is rapidly released, allowing the electron to return to its initial “ground” energy state (Fig. 8.10a). Most of the energy (>95%) is converted into heat; a small amount is reemitted as light (fluorescence). By contrast, for chlorophyll molecules situated within the photosystems of the thylakoid, something entirely different occurs: The absorbed light energy is transferred to another chlorophyll molecule and then on to another (Fig. 8.10b).
8-10
Most of the chlorophyll molecules in the thylakoid membrane function as an antenna: Absorbed light energy is transferred from one chlorophyll molecule to another until it is finally transferred to a specially configured pair of chlorophyll molecules known as the reaction center. As we will see, the reaction center is where light energy is converted into electron transport. This division of labor among chlorophyll molecules was discovered in the 1940s in a series of experiments by the American biophysicists Robert Emerson and William Arnold, who showed that only a small fraction of chlorophyll molecules are directly involved in electron transport (Fig. 8.11). We now know that several hundred antenna chlorophyll molecules are associated with each reaction center. The antenna chlorophylls allow the photosynthetic electron transport chain to operate efficiently. Without the antenna to gather light energy, reaction centers would sit idle much of the time, even in bright sunlight.
FIG. 8.11: Do chlorophyll molecules operate on their own or in groups?
BACKGROUND By about 1915, scientists knew that chlorophyll was the pigment responsible for absorbing light energy in photosynthesis. However, it was unclear how these pigments contributed to the reduction of CO2. The American physiologists Robert Emerson and William Arnold set out to determine the nature of the “photochemical unit” by quantifying how many chlorophyll molecules were needed to incorporate one CO2 molecule into carbohydrate.
EXPERIMENT Emerson and Arnold exposed flasks of the green alga Chlorella to flashes of light of such short duration (10–5 s) that each chlorophyll molecule could be “excited” only once, and the time between flashes was long enough to allow the reactions resulting from this light energy to run to completion. In step 1, they recorded the maximum rate of CO2 uptake by increasing the intensity of the light flashes until the rate could not go any higher. In step 2, they determined the concentration of chlorophyll present in their solution of cells. They then compared the maximum rate of CO2 uptake to the number of chlorophyll molecules.
RESULTS
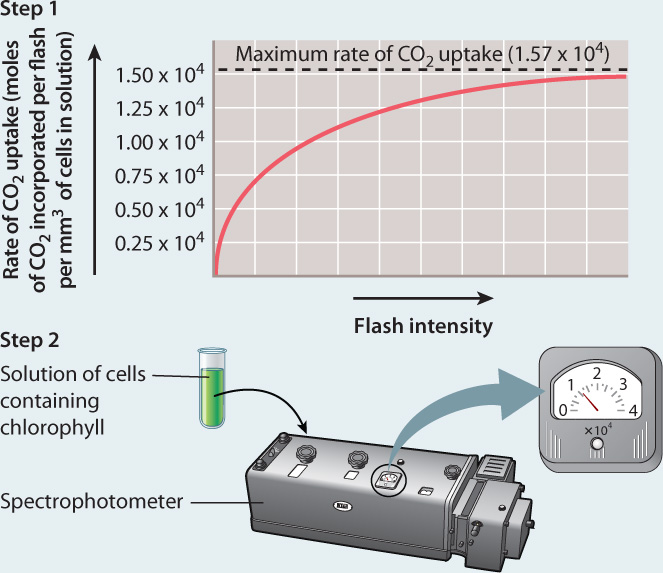
CONCLUSION Because the maximum rate of CO2 uptake per flash was much smaller than the amount of chlorophyll in their flask, Emerson and Arnold concluded that each photochemical unit contains many chlorophyll molecules.
FOLLOW-UP WORK Emerson and Arnold’s work was followed by studies that demonstrated that the photosynthetic electron-transport chain contains two photosystems (photosynthetic units) arranged in series.
SOURCE Emerson, R., and W. Arnold. 1932. “The Photochemical Reaction in Photosynthesis.” Journal of General Physiology. 16:191–205.
The chlorophyll molecules that make up the antenna are precisely spaced so that when a chlorophyll molecule absorbs light it transfers energy, but not electrons, to an adjacent chlorophyll molecule (see Fig. 8.10b). A good analogy for this form of energy transfer is the transfer of vibrational energy between two tuning forks held close together. The transfer of energy between antenna chlorophyll molecules is highly efficient, so little energy is lost as heat, in contrast to what happens with an isolated chlorophyll molecule. Light energy absorbed by the antenna is passed from one chlorophyll molecule to the next until eventually it is transferred to the reaction center, as shown in Fig. 8.12a.
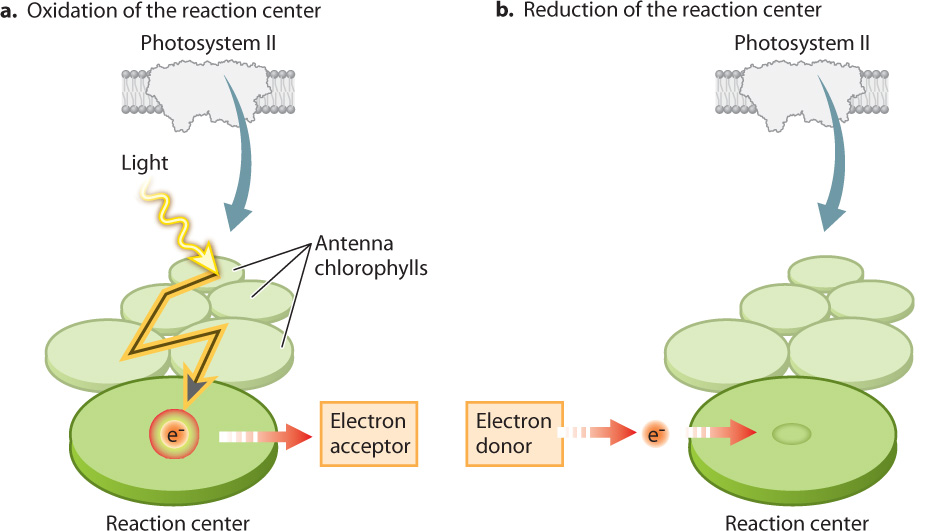
The reaction center chlorophylls have a configuration distinct from that of the antenna chlorophylls. As a result, reaction center chlorophylls are able to transfer both absorbed light energy and an associated high-energy electron to an adjacent molecule that acts as an electron acceptor. When the transfer takes place, the reaction center becomes oxidized and the adjacent electron-acceptor molecule is reduced. The result is the conversion of light energy into a chemical form. This electron transfer sets in motion the light-driven chain of redox reactions that constitute the photosynthetic electron transport chain.
8-11
8-12
Once the reaction center has lost an electron, it is no longer able to absorb light. From a visual perspective, it has been bleached. Thus, for the photosystem to continue contributing electrons to the photosynthetic electron transport chain, another electron must be delivered to take the place of the one that has entered the transport chain (Fig. 8.12b). As we will see below, these replacement electrons ultimately come from water.
Question Quick Check 3
VJ6SzB0ZzNDsDZp+DPric4lDhBJX6a3DRwqyJp9Ezhhjy5BEtOZL4LUNWGVe2Gd/VWPg/NKLWxOu5hdPb8RQ4pNCkSJJHXX16IODOw==8.3.2 The photosynthetic electron transport chain connects two photosystems.
Experiments conducted in the 1940s led to the surprising finding that photosynthetic cells use light energy at only half the maximum efficiency predicted. We now know that the reason that the efficiency is lower than predicted is that the photosynthetic electron transport chain includes not one but two photosystems arranged in series. Two photosystems are necessary to provide enough energy to pull electrons from water and then use them to reduce NADP+.
If you follow the flow of electrons from water, through both photosystems, and on to NADP+, as shown in Fig. 8.13, you can see a large increase in the energy level of the electrons as they pass through each of the two photosystems. You can also see that every other step along the photosynthetic electron transport chain is associated with a small decrease in the energy level of the electrons. Because the overall energy trajectory has an up-down configuration resembling a “Z,” the photosynthetic electron transport chain is sometimes referred to as the Z scheme.
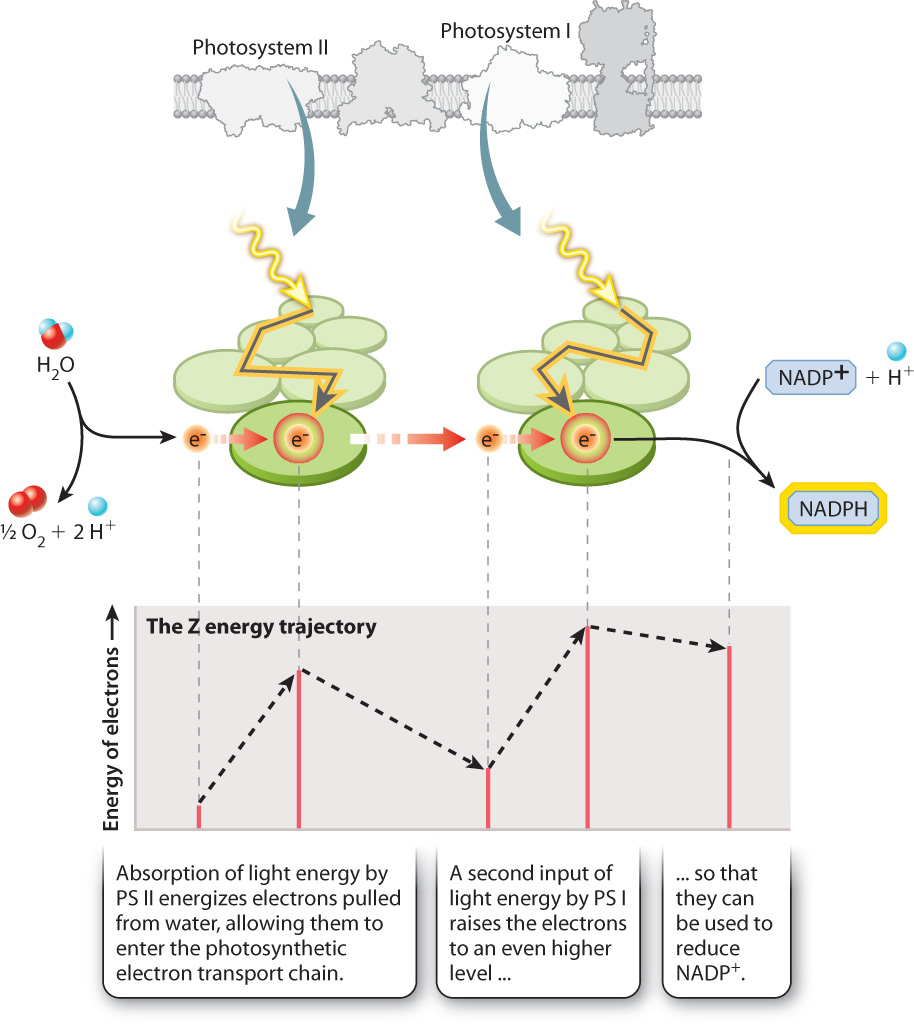
For the two photosystems to work together to move electrons from water to NADPH, they must have distinct chemical properties. Photosystem II supplies electrons to the beginning of the electron transport chain. When photosystem II loses an electron (that is, when it is itself oxidized), it is able to pull electrons from water. In contrast, photosystem I energizes electrons with a second input of light energy so they have enough energy to reduce NADP+. The key point here is that photosystem I when oxidized is not a sufficiently strong oxidant to split water, whereas photosystem II cannot produce electrons with enough energy to form NADPH.
The major protein complexes of the photosynthetic electron transport chain include the two photosystems as well as the cytochrome b6f complex, through which electrons pass between photosystem II and photosystem I (Figure 8.14). Small, relatively mobile compounds convey electrons between these protein complexes. Plastoquinone, a lipid-soluble mobile compound, carries electrons from photosystem II to the cytochrome b6f complex, while plastocyanin, a water-soluble protein, carries electrons from the cytochrome b6f complex to photosystem I by diffusing through the thylakoid lumen. The modular nature of the photosynthetic electron transport chain, interconnected by diffusible elements, enables this pathway to adjust in response to changes in the availability of light.
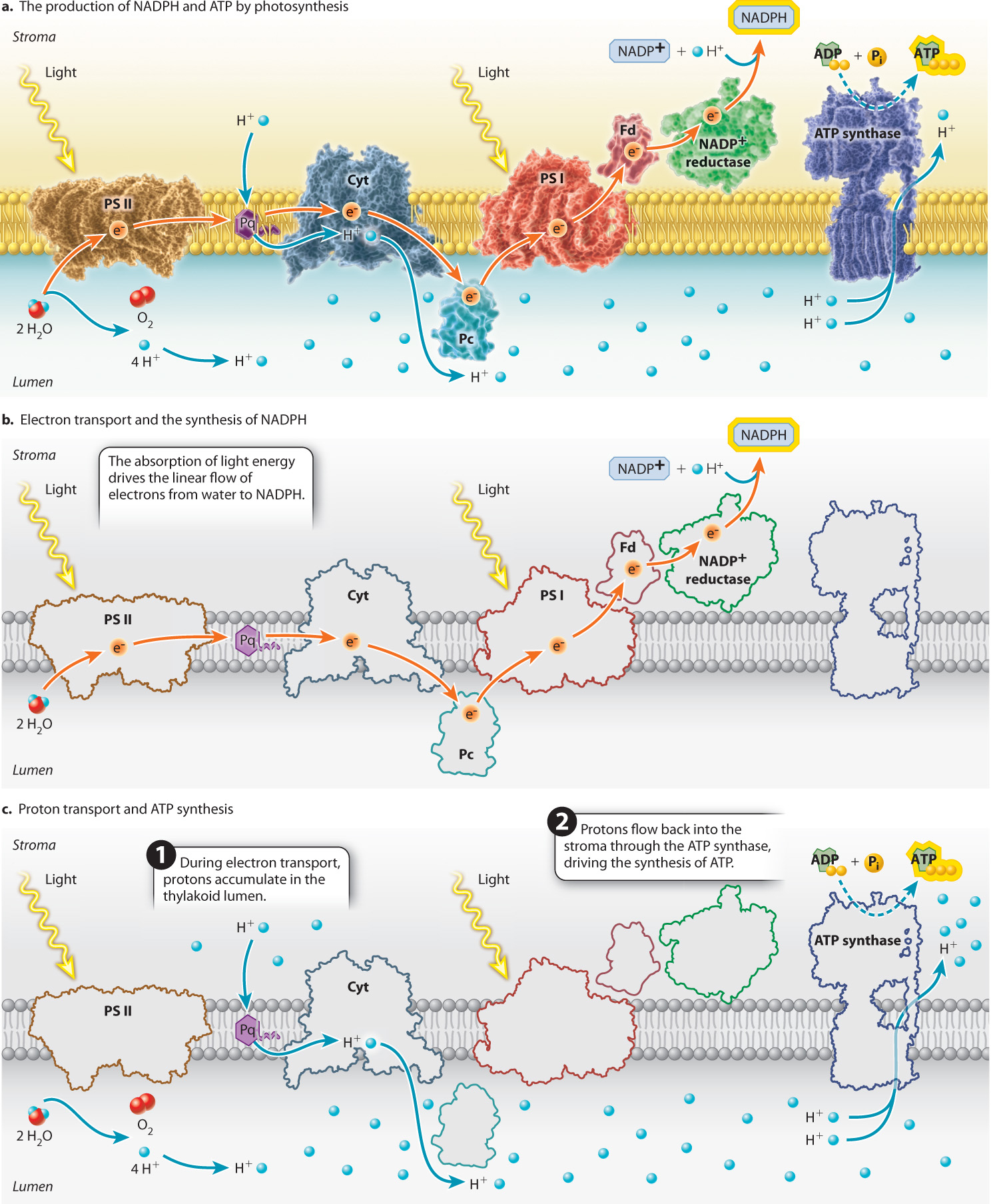
Water, as the electron donor, sits at one end of the photosynthetic electron transport chain, whereas NADP+, the electron acceptor that transports high-energy electrons to the Calvin cycle, sits at the other. NADPH is formed when high-energy electrons are passed from photosystem I to a membrane-associated protein called ferredoxin (Fig. 8.14b). The enzyme ferredoxin–NADP+ reductase then catalyzes the formation of NADPH by transferring two high-energy electrons to NADP+ as well as a proton from the surrounding solution:
NADP+ + 2e– + H+ → NADPH
8-13
8-14
Question Quick Check 4
ZBBPTzLE6qGcVWeYcmcOyDTSqMODalGNbTo/fDaRzvfVrLIyaLvDbDQ+4cgOItiz/xlhr1WYMwdcv0RzI22pW5kkK+BmARPM11eChyd/VWfpRn8I8.3.3 The accumulation of protons in the thylakoid lumen drives the synthesis of ATP.
So far, we have considered only how the photosynthetic electron transport chain leads to the formation of NADPH. However, we know that the Calvin cycle also requires ATP. In chloroplasts as in mitochondria, ATP is synthesized by ATP synthase, a transmembrane protein powered by the movement of protons across the membrane. In chloroplasts, the ATP synthase is oriented such that the movement of protons from the thylakoid lumen to the stroma results in the synthesis of ATP, as shown in Fig. 8.14c.
How do protons accumulate in the thylakoid lumen? Two features of the photosynthetic electron transport chain are responsible for the build-up of protons in the thylakoid lumen. First, the oxidation of water releases protons and O2 into the lumen (Fig. 8.14c). Second, the cytochrome-b6f complex, the protein complex situated between photosystem II and photosystem I, functions as a proton pump. As electrons transit through the cytochrome-b6f complex, some of the energy that is released by each redox reaction is used to drive protons from the stroma side of the thylakoid membrane to the lumen (Fig. 8.14c).
These two mechanisms are quite powerful. When the photosynthetic electron transport chain is operating at full capacity, the concentration of protons in the lumen can be more than 1000 times greater than that in the stroma (equivalent to a difference of 3 pH units). This accumulation of protons on one side of the thylakoid membrane can then be used to power the synthesis of ATP. If offered a pathway through the membrane, protons diffuse from this region of higher concentration and net positive charge to the region of lower concentration and net negative charge in the stroma, releasing energy. The pathway most readily available is through a channel in the ATP synthase. When protons pass through this enzyme, some of the energy that is released is used to drive the synthesis of ATP (Chapter 7).
8.3.4 Cyclic electron transport increases the production of ATP.
Sunlight varies in intensity throughout the day. When light levels are low, light is shared evenly between the two photosystems. However, as light levels increase, the light energy absorbed begins to overwhelm the capacity of the Calvin cycle to make use of NADPH. The danger is that when there is no NADP+ returning from the Calvin cycle to act as the terminal electron acceptor for the photosynthetic electron transport chain, the high-energy electrons can damage the cell.
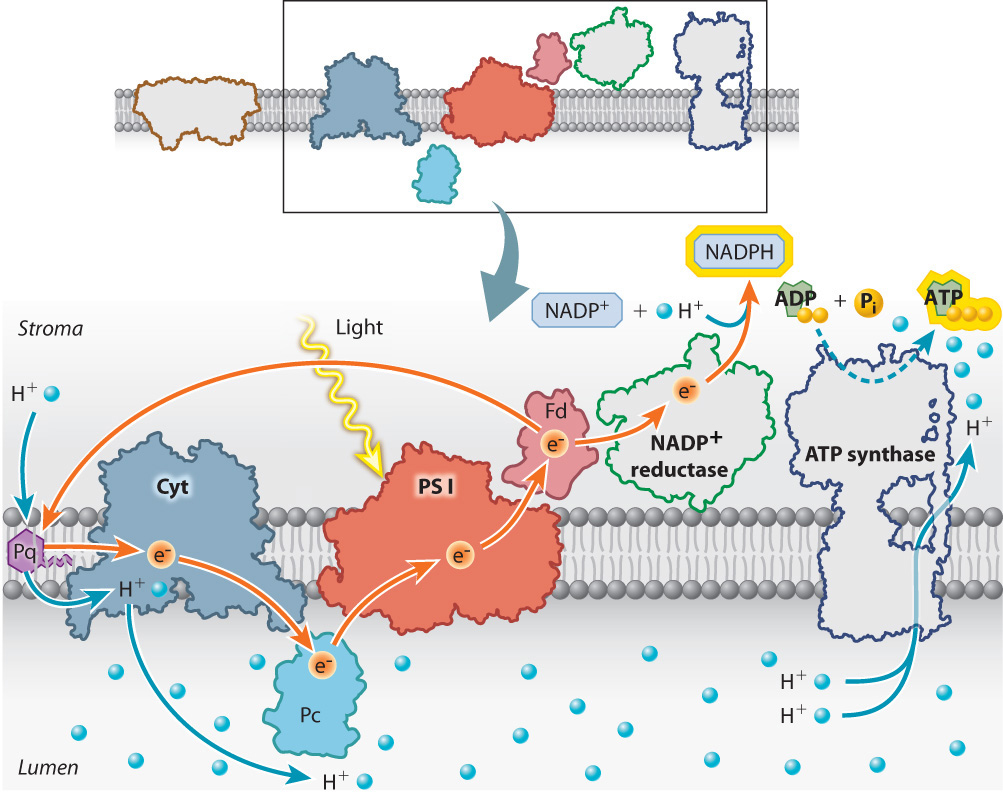
To prevent this from happening, electrons are shunted into an alternative pathway that increases the production of ATP while decreasing the production of NADPH. Electrons from photosystem I are redirected from ferredoxin back into the electron transport chain (Fig. 8.15). These electrons reenter the photosynthetic electron transport chain upstream of the cytochrome-b6f proton pump. Because these electrons eventually return to photosystem I, this alternative pathway is referred to as cyclic electron transport, in contrast to the linear movement of electrons from water to NADPH.
8-15
Cyclic electron transport leads to the production of ATP because as the high-energy electrons from ferredoxin pass through the cytochrome-b6f complex, additional protons are pumped into the lumen. As a result, there are more protons in the lumen that can be used to drive the synthesis of ATP. In addition, a subset of the photosystem II antennae migrate through the thylakoid membrane to become associated with photosystem I. This redistribution of light-harvesting capacity increases the rate of cyclic electron transport relative to linear electron transport, further enhancing the production of ATP.
8.3.5 The spatial organization of the thylakoid membrane contributes to its functioning.
We are now ready to understand how the major components of the photosynthetic electron transport chain are positioned on the thylakoid membrane in a manner consistent with their roles in photosynthesis. To do this, we must revisit the peculiar shape of this photosynthetic membrane, shown in Fig. 8.5. Recall that, despite its convoluted arrangement, the thylakoid membrane forms a single large sac, with the lumen on the inside and the stroma on the outside. The convoluted arrangement of interlinked granal stacks increases the total surface area of the thylakoid that can be accommodated within a single chloroplast. Nevertheless, the tight packing of the granal stacks means that only membranes on the outside of each granal stack and the links between grana are in direct contact with the stroma. The inner folds of the granal stacks have no direct contact with the stroma.
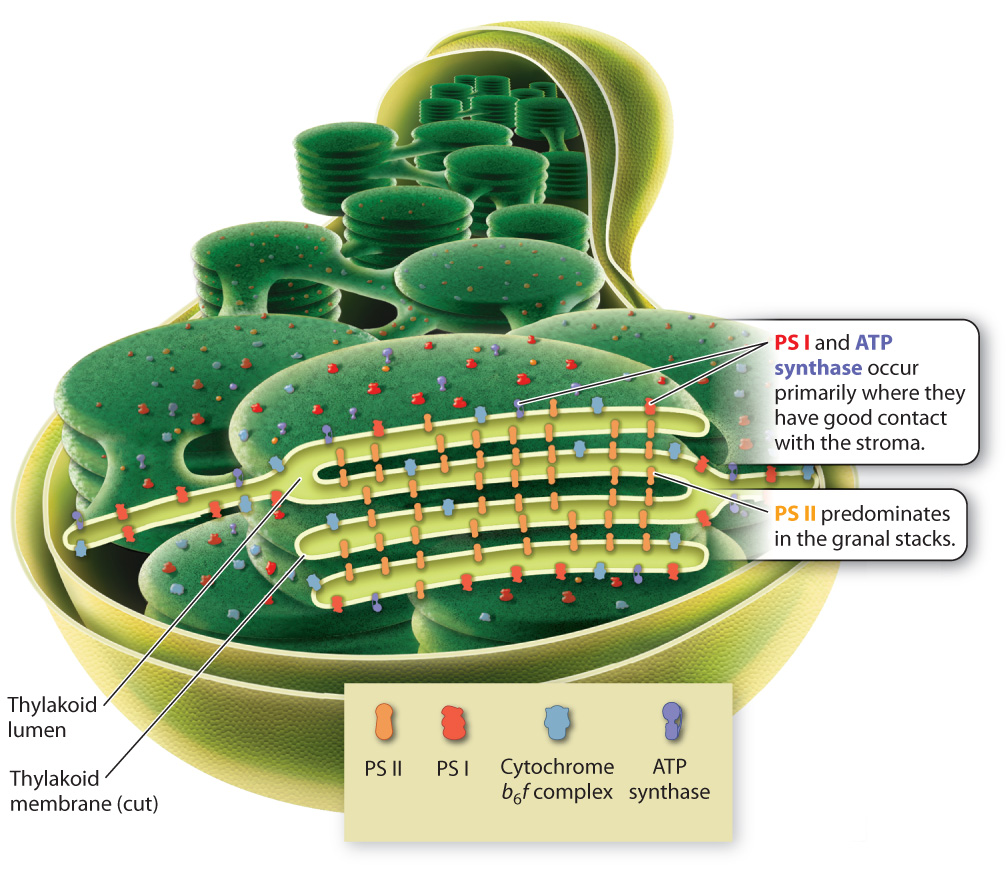
The components of the photosynthetic electron transport chain are distributed in such a way as to make tight packing possible, increasing surface area without sacrificing function. Photosystem I and the ATP synthase are concentrated on the outer regions of the granal stacks (Fig. 8.16). From there they can readily supply NADPH and ATP to the stroma, where these molecules are used to power the Calvin cycle. In contrast, photosystem II is located primarily on the closely packed inner regions of the thylakoid membrane that have little contact with the stroma. The cytochrome-b6f complex, which is located between the two photosystems, has a relatively even distribution.
This distribution of components to different regions is made possible by the presence of the mobile compounds that carry electrons between the major components of the photosynthetic electron transport chain. In particular, the diffusion of the protein plastocyanin through the lumen allows photosystem II and photosystem I to be located in different regions of the thylakoid membrane. The close packing of thylakoid membrane regions helps explain why the thylakoid membrane and the inner mitochondrial membrane look so different, even though they both support electron transport chains.
8.3.6 How did early cells meet their energy requirements?
Case 1 The First Cell: Life's Origins
Photosynthesis is a highly complex process, involving the coordination of many reactions and enzymes. This complexity is the result of several billion years of evolution. The first organisms that were able to draw upon sunlight as an energy source would have been much simpler.
How simple? The earliest reaction centers may have used light energy to drive the movement of electrons from a soluble inorganic electron donor in the surrounding medium to an electron-acceptor molecule within the cell. Reduced iron, Fe2+, is thought to have been abundant in the early ocean, and therefore could have served as the first electron donor. Alternatively, the first forms of light-driven electron transport may have been coupled to the net movement of protons across the membrane, allowing for the synthesis of ATP.
8-16
Similarly, it is unlikely that these first photosynthetic organisms employed chlorophyll, a complex molecule whose biosynthetic pathway contains at least 17 enzymatic steps. Because some of the intermediate compounds leading to chlorophyll are themselves capable of absorbing light energy, perhaps each of these now-intermediate compounds was, at one time, a functional end product used by an early photosynthetic organism. Selection for chemical variants with increased efficiency or the ability to absorb new portions of the visible spectrum may then have led to the further elaboration of this pathway. Selection would have eventually resulted in the chlorophyll pigments that are used by photosynthetic organisms today.