8.4 CHALLENGES TO PHOTOSYNTHETIC EFFICIENCY
The efficient functioning of photosynthesis faces two major challenges. The first is that if more energy is generated than the Calvin cycle can use, excess light energy can damage the cell. The second challenge stems from rubisco’s ability to catalyze the addition of either carbon dioxide or oxygen to RuBP. The addition of oxygen instead of carbon dioxide can substantially reduce the amount of carbohydrate produced.
8.4.1 Excess light energy can cause damage.
Photosynthesis is an inherently dangerous enterprise. Unless the photosynthetic reactions are carefully controlled, molecules will be formed that can damage cells through the indiscriminate oxidization of lipids, proteins, and nucleic acids (Fig. 8.17).
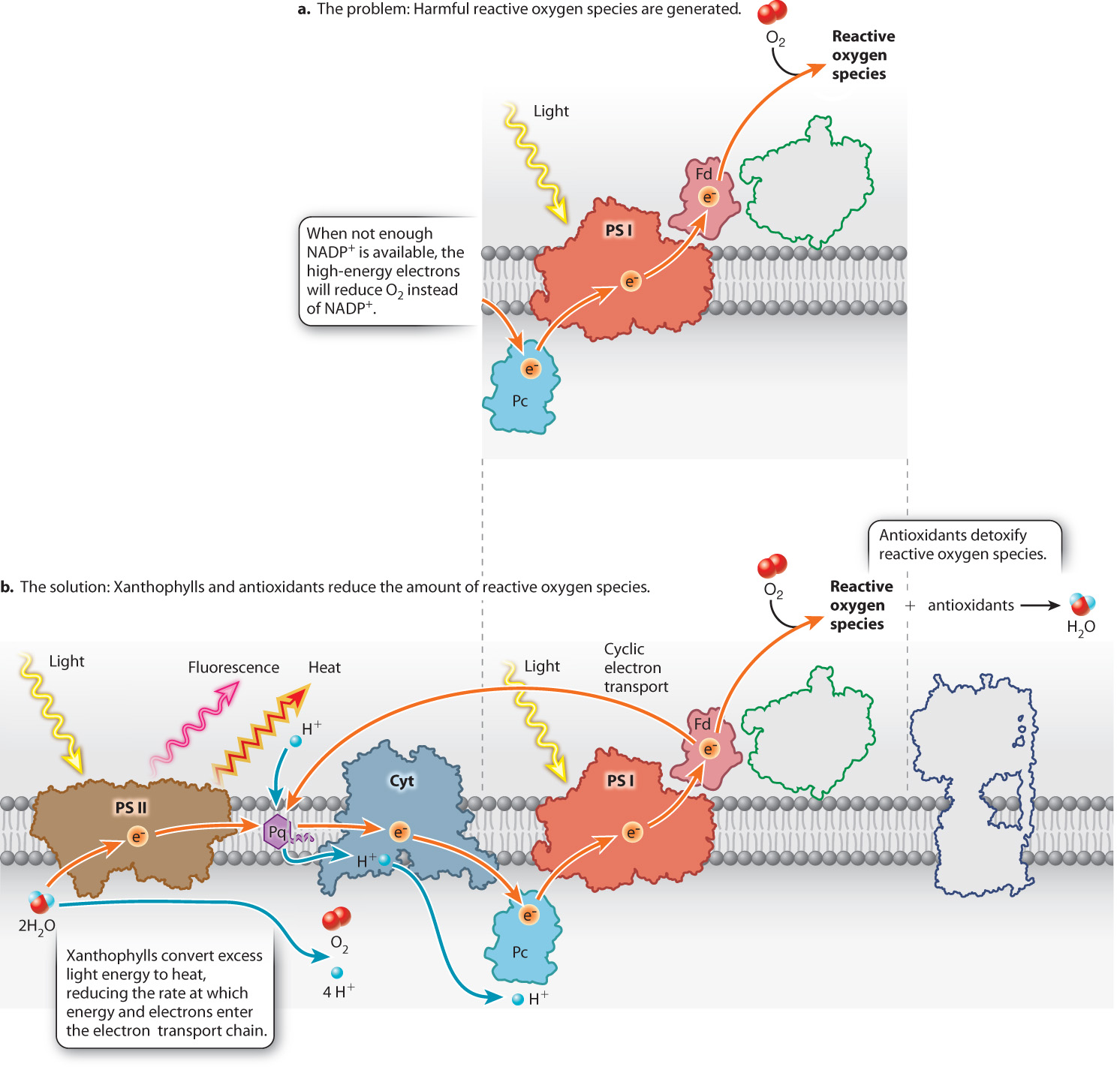
Under normal conditions, the redox reactions that make up the photosynthetic electron transport chain do not allow either absorbed light energy or the resulting high-energy electrons to stray. However, when NADP+ is in short supply, either the absorbed light energy or the energy and the associated electron can be transferred to O2, resulting in the formation of highly reactive forms of oxygen known collectively as reactive oxygen species (Fig. 8.17a).
NADP+ is returned to the photosynthetic electron transport chain by the Calvin cycle’s consumption of NADPH. Thus, any factor that causes the rate of NADP+ regeneration to fall behind the rate of light-driven electron transport can potentially lead to damage. Such an imbalance is likely to occur, for example, in the middle of the day when light intensity is highest. Photosynthetic cells could prevent excess light energy by synthesizing sufficient quantities of Calvin cycle enzymes to process all the energy absorbed by chlorophyll even at full sunlight. This strategy, however, would be energetically expensive because light levels vary dramatically over the course of the day. When light levels are low, such as in the morning and late afternoon, Calvin cycle enzymes would sit idle.
The rate at which the Calvin cycle can utilize NADPH is also influenced by a number of factors that are independent of light intensity. For example, cold temperatures cause the enzymes of the Calvin cycle to function more slowly, but they have little impact on the absorption of light energy by the photosynthetic electron transport chain. On a cold, sunny day, more light energy is absorbed than can be used by the Calvin cycle. Other factors that depress the rate at which the Calvin cycle can function include shortages of nitrogen, which reduces protein levels overall, and of CO2.
Photosynthetic organisms employ two major lines of defense to deal with the stresses that occur when the Calvin cycle cannot keep up with light harvesting (Fig. 8.17b). First among these are chemicals that detoxify reactive oxygen species. Ascorbate (vitamin C), beta-carotene, and other antioxidants are able to neutralize reactive oxygen species. These compounds exist in high concentration in chloroplasts. Some of these antioxidant molecules are brightly colored, like the red pigments found in algae that live on snow shown in Fig. 8.3c. The presence of antioxidant compounds is one of the many reasons that eating photosynthetic tissues is good for your health.
A second line of defense is to prevent reactive oxygen species from forming in the first place. Xanthophylls are yellow-orange pigments that slow the formation of reactive oxygen species by reducing excess light energy. These pigments accept absorbed light energy directly from chlorophyll and then convert this energy to heat (Fig. 8.17b). Photosynthetic organisms that live in extreme environments often appear brown or yellow because of high levels of xanthophyll pigments, as seen in Figs. 8.3a and 8.3b. Plants that lack xanthophylls grow poorly when exposed to moderate light levels and die in full sunlight.
Converting absorbed light energy into heat is beneficial at high light levels, but at low light levels it would decrease the production of carbohydrates. Therefore, this ability is switched on only when the photosynthetic electron transport chain is working at high capacity. The creation of a strong proton gradient across the thylakoid membrane at high light levels activates the enzyme that converts inactive xanthophyll molecules into their light-absorbing form.
Xanthophyll pigments are predominantly associated with photosystem II. When activated by high light, these light-absorbing pigments reduce linear electron transport, but allow cyclic electron transport to continue. Light absorbed by photosystem I thus remains available to power the synthesis of ATP, which can then be used to help repair cellular components that have been damaged by reactive oxygen species.
8-17
8.4.2 Photorespiration leads to a net loss of energy and carbon.
A major challenge to photosynthetic efficiency is the fact that rubisco can add O2 to RuBP instead of CO2. The idea that an enzyme could make a “mistake” may seem surprising, yet, from the point of view of a very large enzyme, CO2 and O2 have a lot in common: They are similar in size, have similar chemical bonds, and are both uncharged. Moreover, O2 is approximately 500 times more abundant in the atmosphere than CO2.
8-18
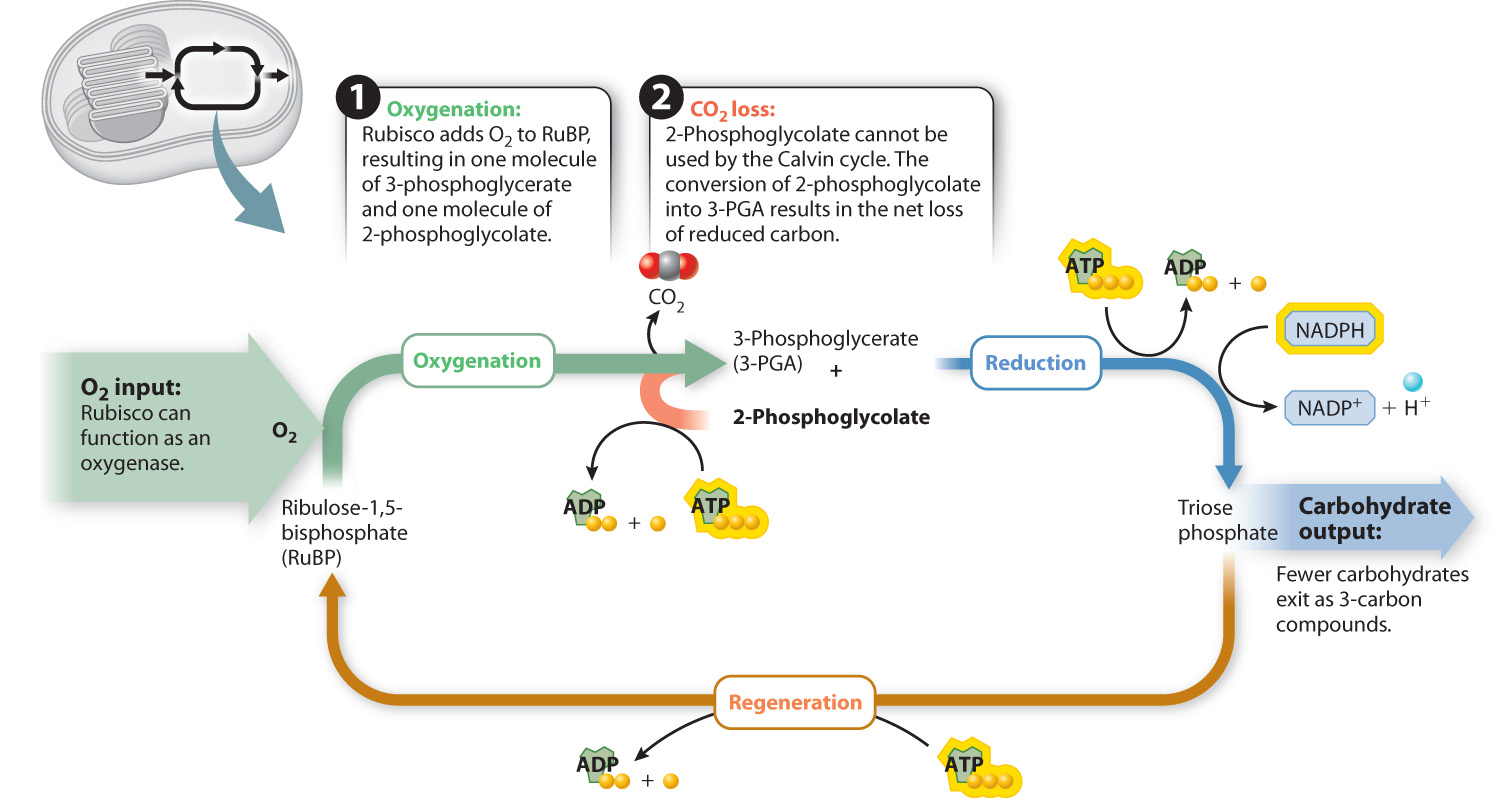
When rubisco adds O2 instead of CO2 to RuBP, the result is one molecule with three carbon atoms (3-PGA) and one molecule with only two carbon atoms (2-phosphoglycolate). The production of 2-phosphoglycolate creates a serious problem because this molecule cannot be utilized by the Calvin cycle either to produce triose phosphate or to regenerate RuBP.
A metabolic pathway to recycle 2-phosphoglycolate is present in photosynthetic cells. However, this pathway is not able to return all of the carbon atoms in 2-phosphoglycolate to the Calvin cycle; some are released as CO2. Because the overall effect of oxygenation is a release of CO2 in the presence of light, this process is referred to as photorespiration (Fig. 8.18). However, unlike respiration, which produces ATP, photorespiration actually consumes ATP. In photorespiration, ATP is used to drive the reactions that convert a portion of the carbon atoms in 2-phosphoglycolate into 3-PGA, which can reenter the Calvin cycle. Thus, photorespiration represents a net energy drain on two accounts: First, it results in the oxidation and loss, in the form of CO2, of carbon atoms that had previously been incorporated and reduced by the Calvin cycle, and second, it consumes ATP.
Rubisco plays a key role in photosynthesis, and yet it is an enzyme that makes mistakes, “confusing” O2 and CO2. What accounts for its evolutionary success? A partial answer is that rubisco first evolved long before oxygen appeared in Earth’s atmosphere. Still, why would photorespiration persist in the face of what must be strong evolutionary pressure to reduce or eliminate the unwanted oxygenation reaction?
The difficulty is that for rubisco to favor the addition of CO2 over O2 requires a high degree of selectivity, and the price of selectivity is speed. The better rubisco is at discriminating between CO2 and O2, the slower its catalytic rate. Nowhere is this trade-off more evident than in land plants, whose photosynthetic cells acquire CO2 from an O2-rich and CO2-poor atmosphere. The rubiscos of land plants are highly selective, but incredibly slow, with catalytic rates on the order of three reactions per second. To put this rate in perspective, it is not uncommon for metabolic enzymes to achieve a catalytic rate of a hundred thousand reactions per second.
This trade-off between selectivity and speed is a key constraint for photosynthetic organisms. For land plants, rubisco’s low catalytic rate means that photosynthetic cells must produce huge amounts of this enzyme; as much as 50% of the total protein within a leaf is rubisco. At the same time, the low concentration of CO2 in the atmosphere (0.0385%) relative to O2 (21%) means that as much as one-quarter of the reduced carbon formed in photosynthesis can be lost via photorespiration.
Question Quick Check 5
fbzKco2VhxqY8ir0QHEh0lIOKGVqFh4r1vrF+oXhxGlGg6Yfsz48sbJYHXgWXJcKQviVisaHmi/T5ErReBuE0lN5QHmjW+qZP/r85RYnPlhwXEyS8-19
8.4.3 Photosynthesis captures just a small percentage of incoming solar energy.
Typically, only 1% to 2% of the sun’s energy that lands on a leaf ends up in carbohydrates. Does this mean that photosynthesis is incredibly wasteful? Or is this process, the product of billions of years of evolution, surprisingly efficient? This is not an idle question. The effects of rising CO2 concentrations on Earth’s climate, the search for a renewable, carbon-neutral fuel to power our transportation sector, and the agricultural demands of our skyrocketing human population all point to photosynthesis as a metabolic process relevant to solving some of our most pressing global issues.
Photosynthetic efficiency is typically calculated relative to the total energy output of the sun (Fig. 8.19). However, only visible light has the appropriate energy levels to produce the high-energy electrons required by the photosynthetic electron transport chain. Most of the sun’s output (~60%) is not absorbed by chlorophyll and thus cannot be used in photosynthesis. In addition, leaves are not perfect at absorbing visible light—about 8% is either reflected or passes through the leaf. Finally, even under optimal conditions, not all of the light energy absorbed by chlorophyll can be transferred to the reaction center and instead is given off as heat (also ~8%). As we have seen, when light levels are high, excess light is actively converted into heat by xanthophyll pigments.
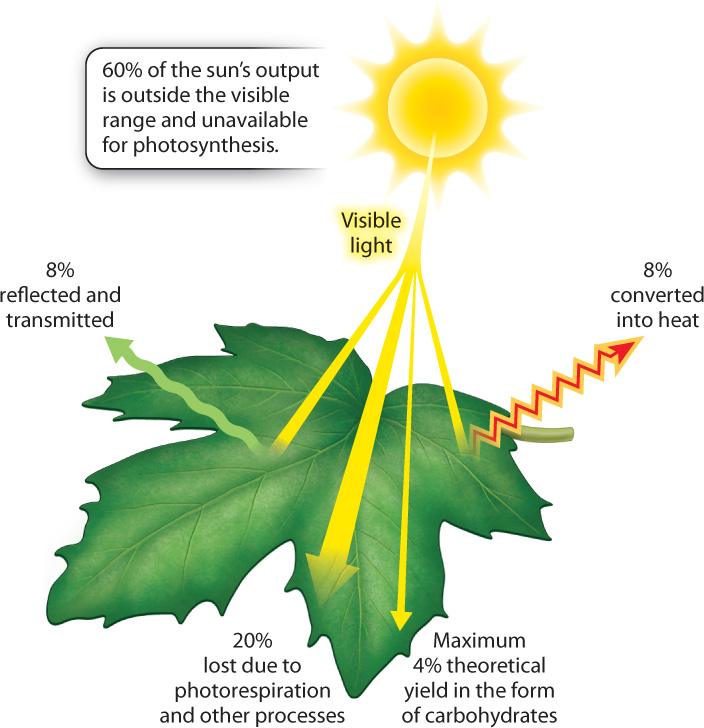
The photosynthetic electron transport chain therefore captures at most about 24% of the sun’s usable energy arriving at the surface of a leaf (100% – 60% – 8% – 8% = 24%). While this number may appear low, it is on a par with the number of high-performance photovoltaic cells in solar panels, which convert sunlight into electricity. This comparison is even more impressive when you consider that photosynthetic organisms must build and maintain all their biochemical machinery. However, energy is lost at another step as well. The incorporation of CO2 into carbohydrates results in considerable loss in free energy, equivalent to ~20% of the total incoming solar radiation. Much of this loss in free energy is due to photorespiration.
In total, therefore, the maximum energy conversion efficiency of photosynthesis is calculated to be around 4% (24% – 20%). Efficiencies achieved by real plants growing in nature, however, are typically much lower, on the order of 1% to 2%. In Chapter 29, we explore the many factors that can constrain the photosynthetic output of land plants, and see how some plants have evolved ways to minimize losses in productivity due to drought and photorespiration.
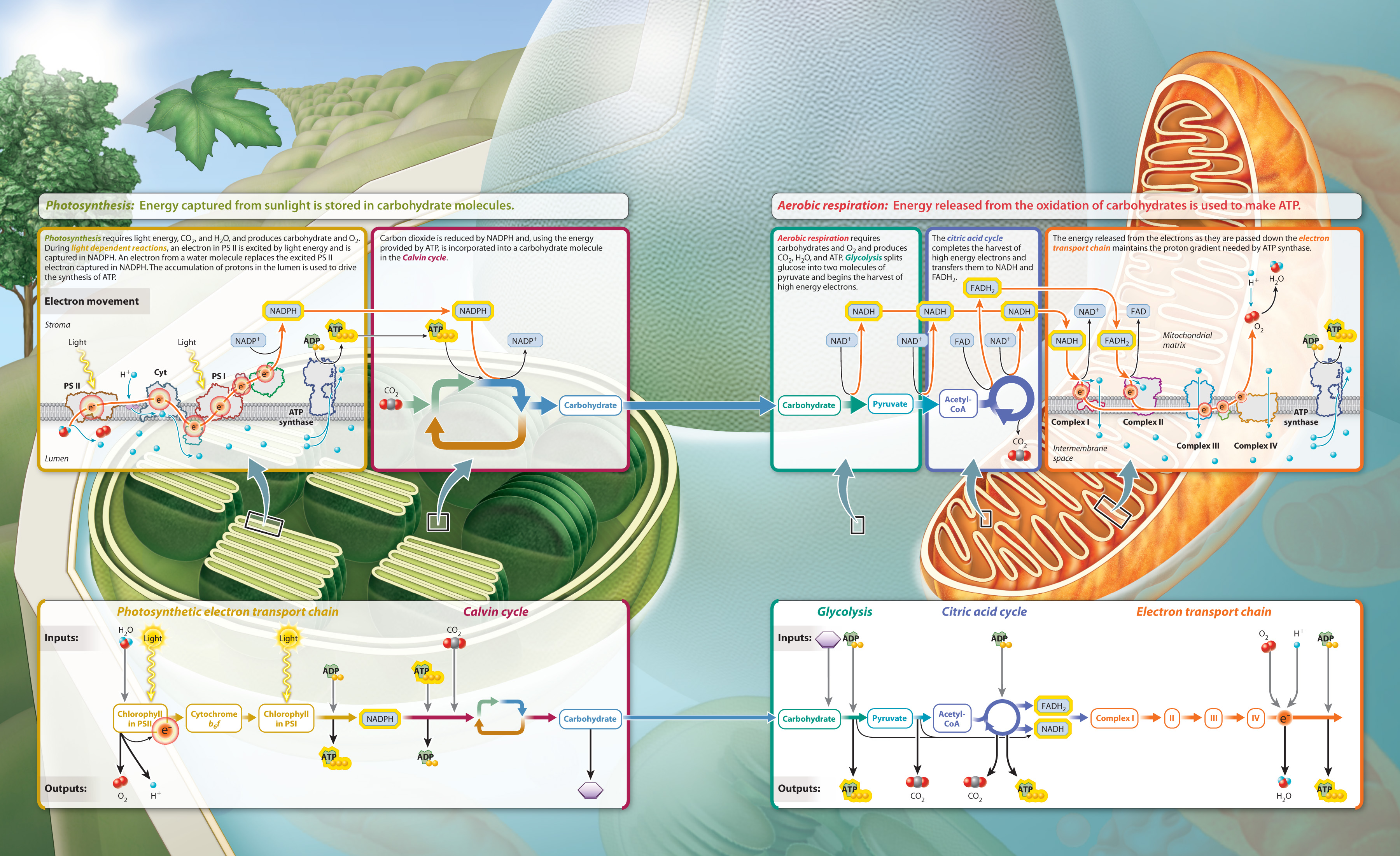
Cellular respiration and photosynthesis are complementary metabolic processes. Cellular respiration breaks down carbohydrates in the presence of oxygen to supply the energy needs of the cell, producing carbon dioxide and water as byproducts, while photosynthesis uses carbon dioxide and water in the presence of sunlight to build carbohydrates, releasing oxygen as a byproduct. We summarize the two processes in Fig. 8.20.
Question Quick Check 6
99eqmZ49mm/sh/i2LLnu/CXbYcOnsnQb5dxRnl37/RwSamHzh/k0JO4cilrjE/9EJ2mOANHPSqZalpGD76hIEcr1Gw+XOW/74gl1u8gQwLuK+5v88j9ZCpYiLfoYU5m8eZB6huvXagtDcK5O/dfFRKDdhhY=8-20
8-21
8-22
Visual Synthesis: Harnessing Energy: Photosynthesis and Cellular Respiration
Click the image below to view an enlarged version in a new window.