9.4 SIGNAL TRANSDUCTION, RESPONSE, AND TERMINATION
In this section, we focus on what happens after a signaling molecule binds to its receptor and flips a molecular switch. Although the molecular switches are different, the subsequent steps are similar. Following the binding of a ligand and receptor activation, most signaling pathways involve signal transduction and amplification, a cellular response, and termination of the signal. We examine the signaling pathways activated by G protein-coupled receptors, receptor kinases, and ligand-gated ion channels. We also consider the role of signal transduction in cancer and how different signaling pathways intersect with one another.
9.4.1 Signals transmitted by G protein-coupled receptors are amplified and regulated at several steps.
Signaling by G protein-coupled receptors is widespread, and its effects are diverse. The receptors are highly conserved evolutionarily and are found in virtually every eukaryotic organism. In humans, more than 900 different G protein-coupled receptors have been found. Signaling through these receptors makes possible our senses of sight, smell, and taste. They also enable the cells that express them to respond to a number of different hormones. Despite this great variability in the nature of the signals, the signal transduction events triggered by these receptors are remarkably similar.
9-9
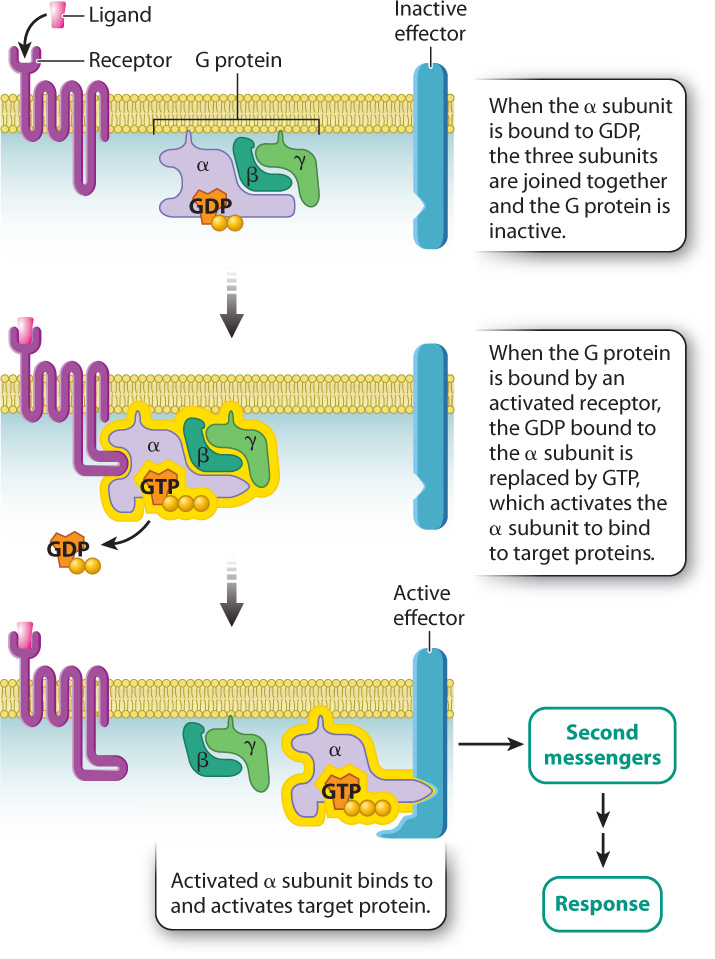
As we saw in section 9.3, G protein-coupled receptors are transmembrane proteins that bind signaling molecules. These receptors are positioned in proximity to G proteins, which are located on the cytoplasmic side of the plasma membrane. When a signaling molecule binds to the receptor, the cytoplasmic domain of the receptor binds to and activates the nearby G protein by exchanging GDP with GTP. The activated G protein goes on to activate additional proteins in the signaling pathway.
G proteins are composed of three subunits called the α (alpha), β (beta), and γ (gamma) subunits (Fig. 9.8). The α subunit is the part of the G protein that binds to either GDP or GTP. When the α subunit is bound to GDP, the three subunits are joined and the G protein is inactive. When the G protein is bound by an activated receptor, the GDP bound to the α subunit is replaced by GTP, which causes the α subunit to separate from the β and γ subunits. The isolated GTP-bound α subunit is now active and able to bind to specific target proteins in the cell, activating them in turn.
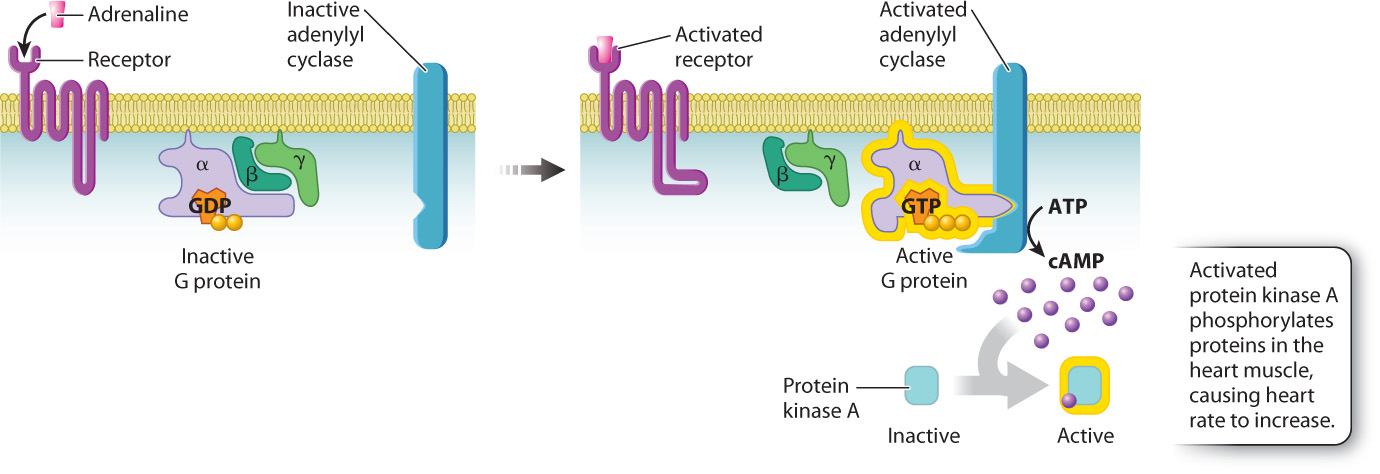
Let’s consider an example. You’re startled or you’re scared, and you experience that strange feeling in the pit of the stomach and rapid heartbeat that we’ve all felt at one time or another. This effect, which accompanies fright or other forms of stress, is mediated by adrenaline, a hormone released by the adrenal glands that binds to a G protein-coupled receptor. When adrenaline binds to its G protein-coupled receptor on cardiac muscle cells, GDP in the G protein is replaced by GTP and activates the G protein. The GTP-bound α subunit of the activated G protein then binds to and activates an enzyme in the cell membrane called adenylyl cyclase. Adenylyl cyclase converts the nucleotide ATP into cyclic AMP, abbreviated as cAMP. This molecule is one of several intermediate, cytosolic signaling molecules known as second messengers (adrenaline being the first messenger). Once formed, cAMP binds to and activates another enzyme, a kinase called protein kinase A, or PKA. The phosphorylation of specific proteins in the heart muscle by PKA causes the rate of contraction to increase (Fig. 9.9). As long as adrenaline is bound to its receptor, the heart rate remains high, resulting in increased blood flow to the brain and skeletal muscles needed to deal with the stress.
A little adrenaline goes a long way as a result of signal amplification. A single receptor bound to adrenaline can activate several individual G protein molecules and continues to do so until the adrenaline leaves the binding site of the receptor. Every one of the activated G protein α subunits binds to an adenylyl cyclase molecule and, as long as the α subunit remains bound, the enzyme continues to generate cAMP. Every cAMP molecule binds to an available protein kinase A molecule, and likewise, as long as cAMP remains bound, the kinase continues to phosphorylate its target proteins. These sequential molecular changes in the cytosol amplify the signal so that a very small amount of signaling molecule has a large effect on a responding cell.
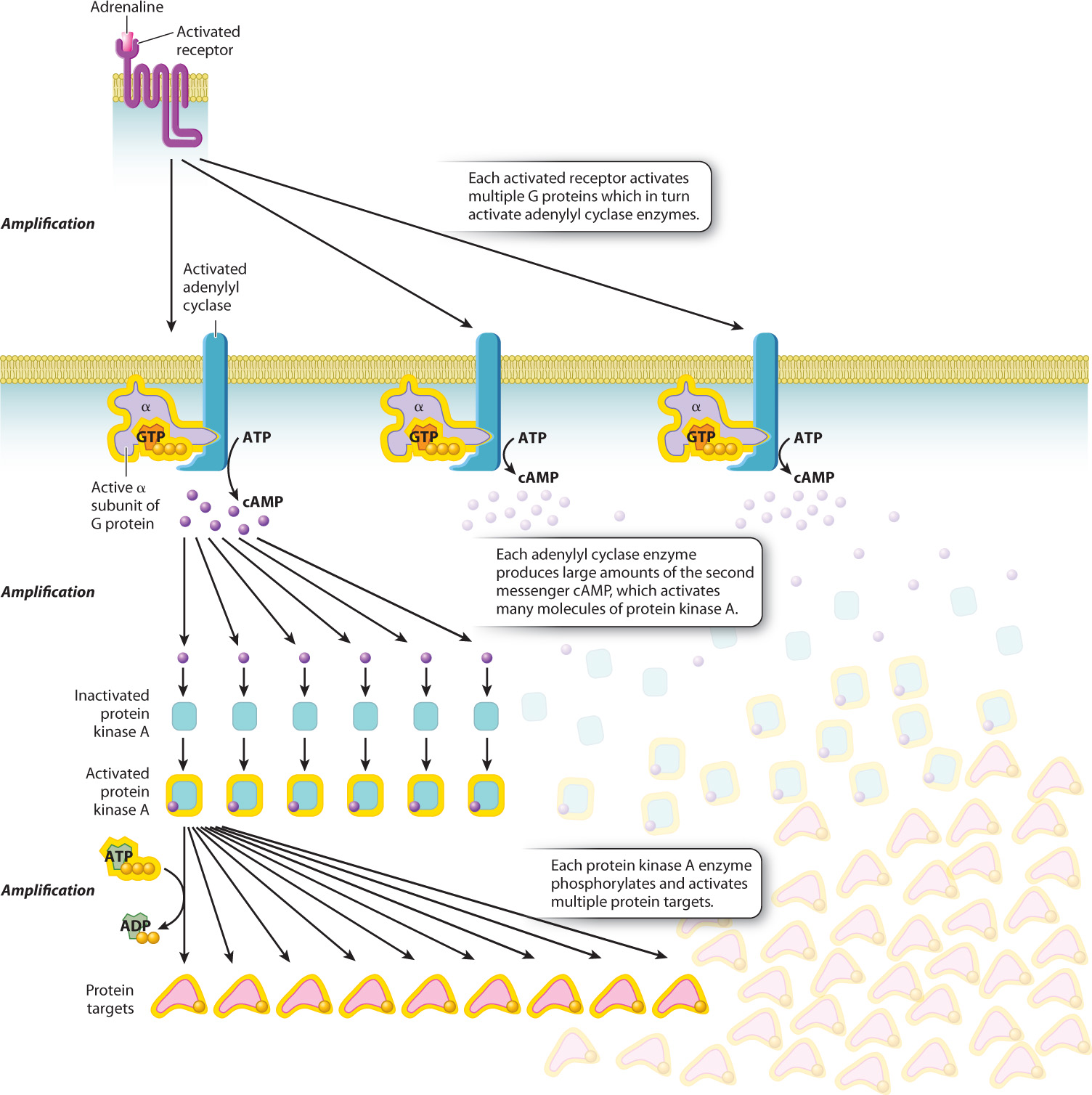
In this example, the adrenaline signal is amplified in three places: (1) Each receptor activates multiple G proteins; (2) each molecule of adenylyl cyclase produces large amounts of the second messenger cAMP; and (3) each protein kinase A molecule activated by cAMP activates multiple protein targets by phosphorylation (Fig. 9.10).
9-10
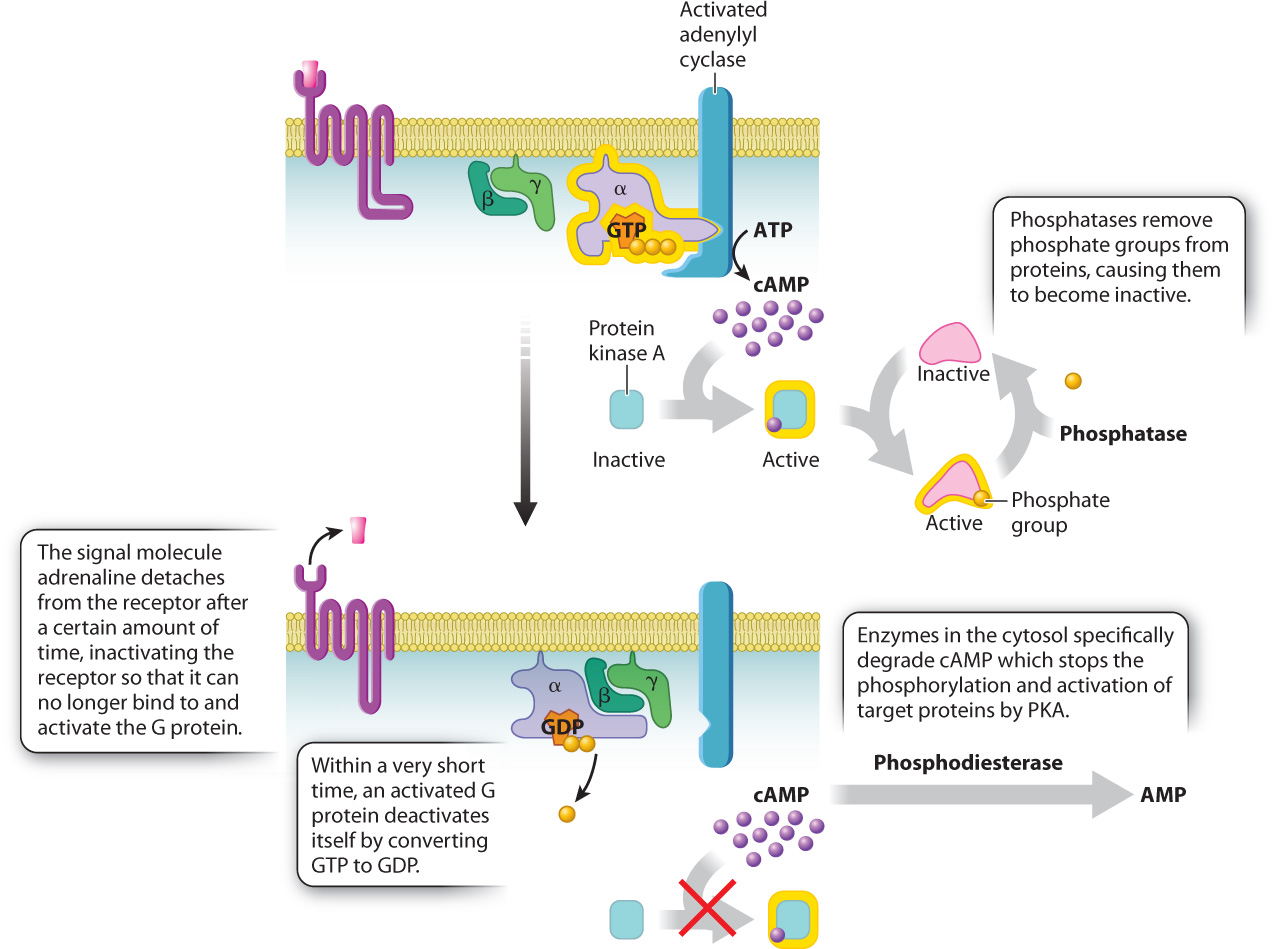
After a good scare, we eventually calm down and our heartbeat returns to normal. This change means that the signal initiated by adrenaline has been terminated. Let’s look at how this happens (Fig. 9.11). First, most ligands, including adrenaline, do not bind to their receptors permanently. The amount of time a signaling molecule remains bound to its receptor depends on how tightly the receptor holds on to it, a property called binding affinity. Once adrenaline leaves the receptor, the receptor reverts to its inactive conformation and no longer activates adjacent G proteins. If the concentration of adrenaline in the system is high enough, then as soon as one adrenaline molecule leaves the receptor, another takes its place and reactivates the receptor. However, once the concentration of adrenaline returns to normal low levels, the receptors are no longer continually occupied and they become inactive.
9-11
Even once a receptor has been turned off, a signal will continue to be transmitted unless all the other components of the signaling pathway are also inactivated. In our example of the adrenaline-signaling pathway, a second place where the signal is terminated is at the G protein itself (Fig. 9.11). As we saw earlier, the α subunit is able to bind to and activate other proteins only when it is bound to GTP. G proteins have enzymatic activity, and they are able to catalyze the hydrolysis of GTP to GDP and inorganic phosphate. This means that an active, GTP-bound α subunit in the “on” position automatically turns itself “off” by converting the GTP to GDP. In fact, the α subunit converts GTP to GDP almost as soon as a molecule of GTP binds to it. Thus, a G protein is able to activate adenylyl cyclase, and adenylyl cyclase is able to make cAMP only during the very short time it takes the α subunit to convert its bound GTP to GDP, a timescale on the order of seconds. Without an active receptor to generate more active G protein α subunits, transmission of the signal quickly comes to a halt.
Farther down the pathway, enzymes shut down other components. An enzyme converts the second messenger cAMP to AMP, which inactivates protein kinase A. Phosphatases remove the phosphate groups, inactivating proteins targeted by protein kinase A (Fig. 9.11). In fact, most signaling events counteract kinases and phosphatases at one or more points in the pathway as a means of increasing or decreasing the response of the cell to the signal.
Question Quick Check 3
2Pa8RoKb3XoH7bSJ7c7yi9Tc9F8WYZjy3wPVdSbbp8mJ2o59bf+57zKhCs9BsfNawg0D1eFvon7tGPrSG1AkvP5pyMX7h0f7Y1w/uuVs/Bo=9.4.2 Receptor kinases phosphorylate each other and activate intracellular signaling pathways.
Like the communication that takes place through G protein-coupled receptors, signaling through receptor kinases causes cells to respond in many ways. During embryonic development, receptor kinase signaling is responsible for the formation and elongation of structures called limb buds that eventually become our arms and legs. As adults, insulin signaling through its receptor kinase enables virtually every cell in our body to transport glucose across the plasma membrane into the cytosol. When we cut a finger, platelet-derived growth factor is released from platelets in the blood and binds to its receptor kinase on the surface of cells at the site of the wound, where it triggers the cell division necessary to repair the damaged tissue.
9-12

Signaling through receptor kinases takes place in most eukaryotic organisms, and the structure and function of these receptors has been remarkably conserved evolutionarily. A receptor kinase called Kit has been studied in a number of animal models. In vertebrates, signaling through the Kit receptor kinase is important for the production of pigment in skin, feathers, scales, and hair. The conserved function of this receptor is obvious in individuals with mutations in the kit gene, as shown in Fig. 9.12. (By convention, the name of a protein, like Kit, is capitalized and in roman type. The name of the gene that encodes the protein, like kit, is italicized and all lower case.) As you can see from Fig. 9.12, mammals, reptiles, birds, and fish with a mutation in the kit gene have a remarkably similar appearance.
Let’s take a look at how receptor kinases work. The cytoplasmic portion of these receptors is a kinase, that is, an enzyme that phosphorylates other molecules. When a signaling molecule binds to the extracellular portion of the receptor, a conformational change causes the receptor to partner up with another receptor kinase bound to another molecule of the same ligand in a process called dimerization. Dimerization activates the cytoplasmic kinase domains of the paired receptors, causing them to phosphorylate each other at multiple sites on their cytoplasmic tails (Fig. 9.13). The addition of these phosphate groups provides places on the receptor where other proteins bind and become active.
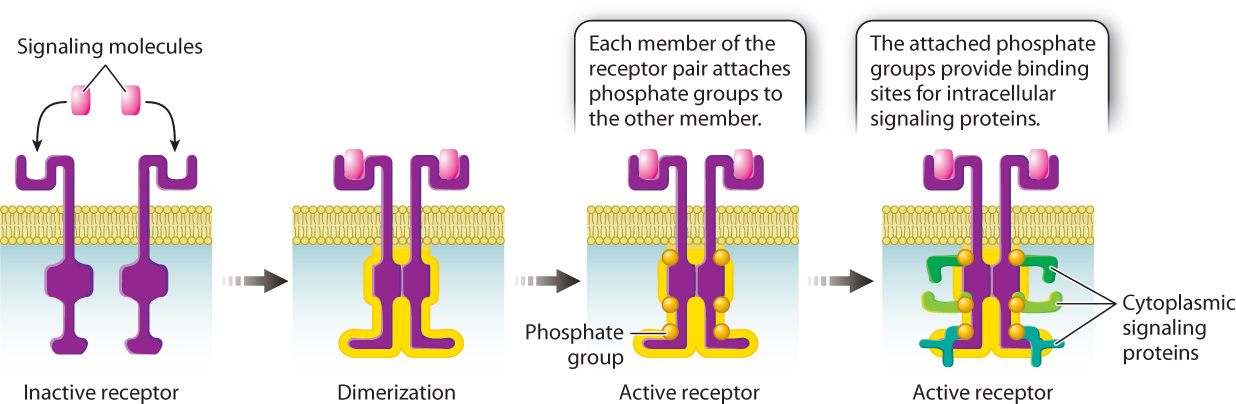
9-13
Signaling through receptor kinases follows the same basic sequence of events that we saw in signaling though G protein-coupled receptors. Binding of the ligand activates the receptor, which causes a set of cytoplasmic proteins to become sequentially activated, and, since many of these proteins are enzymes, the signal is amplified. And as we saw earlier in the G protein-coupled pathway, intracellular signaling proteins in receptor kinase pathways are also activated by GTP binding and phosphorylation, and are inactivated by GTP hydrolysis to GDP and dephosphorylation by phosphatases.
Now let’s look at an example of signaling through a receptor kinase pathway. Think about the last time you got a paper cut. The cut likely bled for a minute or two, and then the bleeding stopped. If the cut was small, it probably healed completely in a week or so. PDGF has a lot to do with getting the healing process started. When platelets in the blood encounter damaged tissue, they release a number of proteins, including PDGF. PDGF is the signaling molecule that binds to PDGF-specific receptor kinases on the surface of cells at the site of a wound, causing the receptors to dimerize and become active. The activated receptors then recruit and activate additional signaling proteins present in the cytosol.
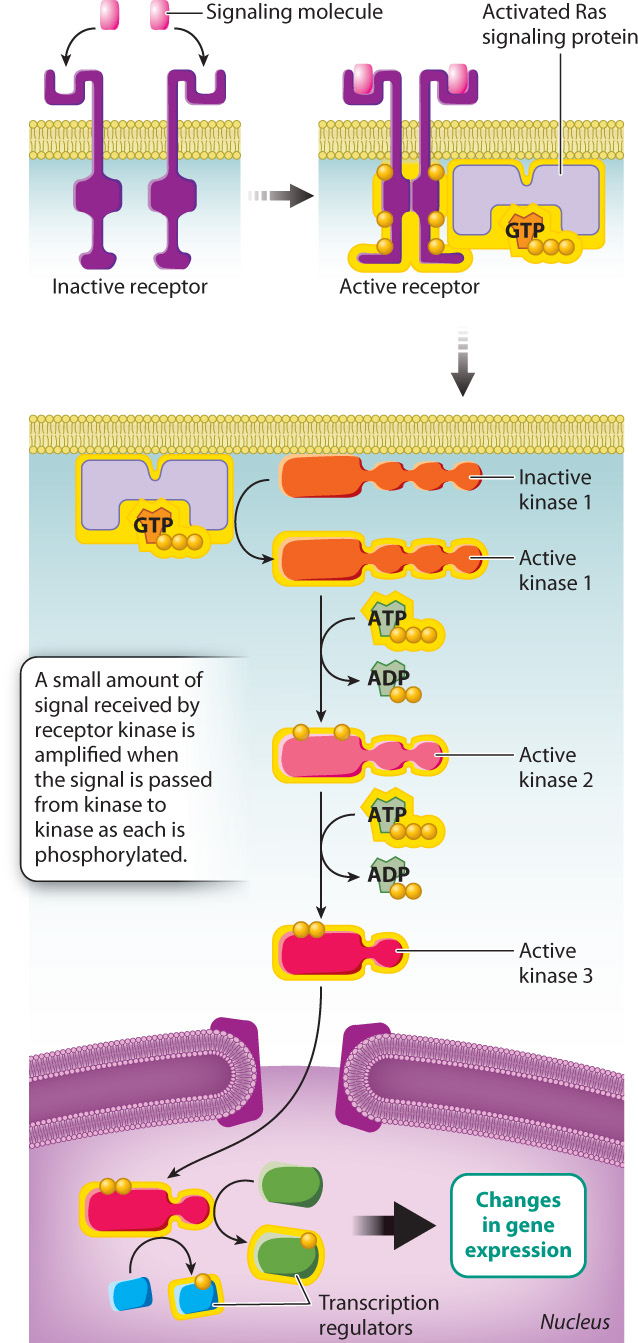
One of these cytoplasmic signaling proteins is Ras, which is very similar to the α subunit of G proteins. In the absence of a signal, Ras is bound to GDP and is inactive. However, when Ras binds to an activated receptor kinase, it releases GDP and binds GTP to become active. Activated GTP-bound Ras triggers the activation of a series of kinases that together are called the mitogen-activated protein kinase pathway, or, for short, the MAP kinase pathway (Fig. 9.14). The final activated kinase in the series enters the nucleus, where it phosphorylates its target proteins. Some of these proteins include regulators of transcription that switch on genes needed for the cell division so that your paper cut can heal.
The signals received by receptor kinases are amplified as the signal is passed from kinase to kinase. Each phosphorylated kinase in the series activates multiple molecules of the downstream kinase, each of which in turn activates many molecules of another kinase still farther downstream. In this way, a very small amount of signaling molecule in the environment (PDGF, in our example) can cause a large-scale response in the cell.
Receptor kinase signaling is also reversed or terminated by the same basic mechanisms that are at work in G protein-coupled receptor pathways. For example, protein phosphatases play important roles in the inactivation of receptor kinases and the other enzymes of the MAP kinase pathway. Furthermore, Ras is a GTPase, just like the G protein α subunit. Shortly after Ras binds to GTP and becomes active, Ras converts GTP to GDP, making it inactive. Without an active receptor kinase to generate more active Ras, activation of MAP kinase pathway components stops.
9.4.3 Ligand-gated ion channels alter the movement of ions across the plasma membrane.
Let’s turn our attention now to the third group of receptors, the ligand-gated ion channels. Instead of activating cytosolic enzymes, these receptors function as ion channels to regulate the permeability of the cell membrane to ions. Nevertheless, the same principles of cell communication apply here as well: A signal binds to a receptor, which is activated and causes a cellular response until the signal is terminated.
9-14
There are many examples of ligand-gated ion channels, each specific for one or more different ions. Ligand-gated ion channels are particularly important in neurons and muscle cells. The plasma membranes of neurons and muscle cells are often referred to as “excitable membranes” because of the dramatic changes that take place in the membrane and in the entire cell when ions, especially sodium ions (Na+), are permitted to flow into the cell. At rest, almost all of the Na+ channels of neurons and muscle cells are closed, and in fact, these cells expend large amounts of ATP to pump Na+ out of the cell. As a result, the concentration of Na+ inside the cell is very low compared to its concentration outside the cell.
The difference in Na+ concentration also contributes to a difference in electrical charge across the plasma membrane, called the membrane potential. At rest, the inside of the cell has a negative charge relative to the outside. This charge difference is in part the result of the large amount of positively charged sodium ions outside the cell and the high concentration of negatively charged ions and proteins in the cytosol. When a signaling molecule released by a neuron binds a ligand-gated Na+ channel, the channel opens, allowing Na+ to move down its concentration gradient into the cell. The influx causes a drastic decline in the charge difference between the outside and inside of the cell. In turn, this change in membrane potential causes the cell to respond: The neuron responds by sending a nerve impulse, and the muscle cell responds by contracting.
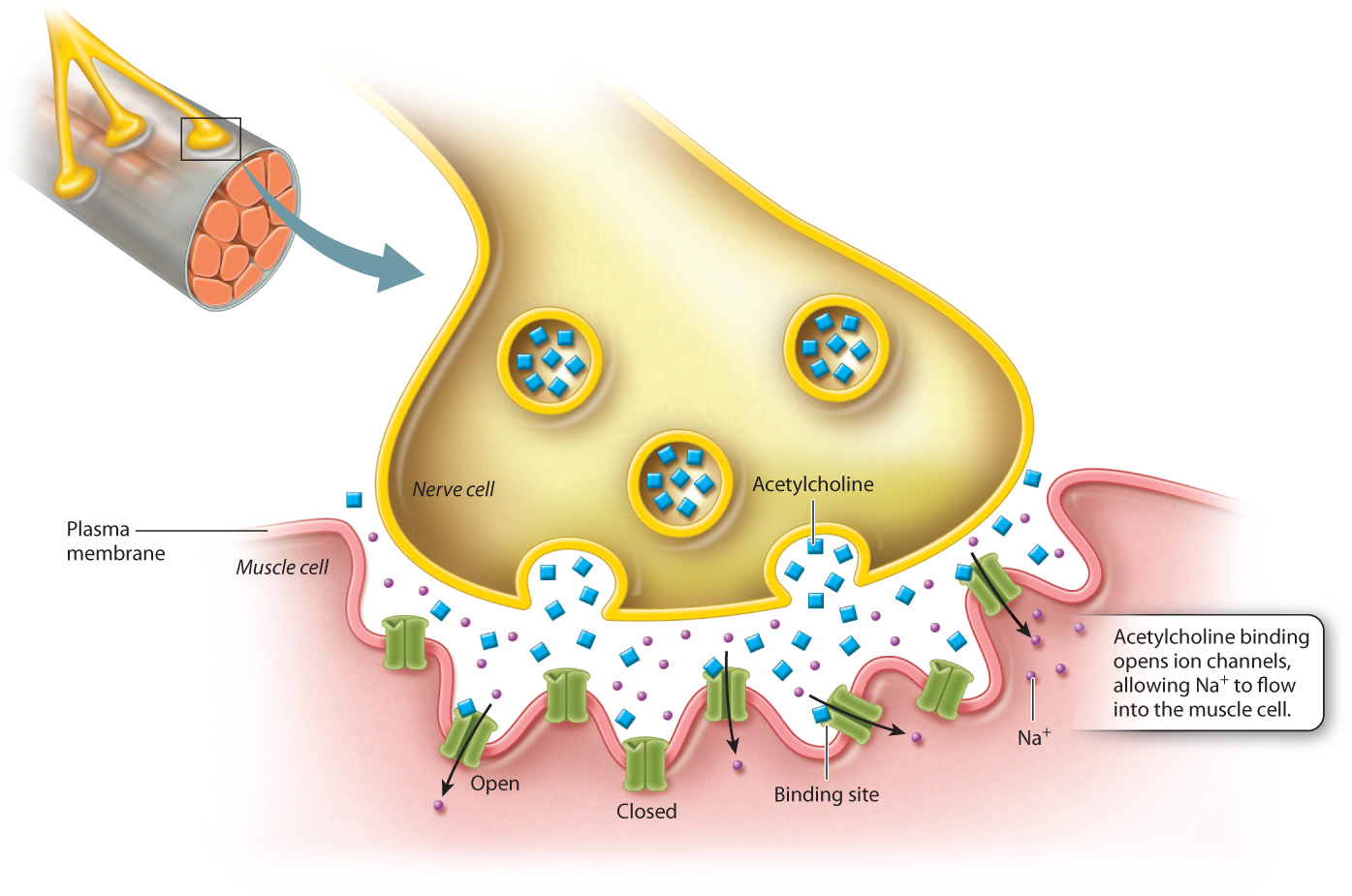
Let’s look more closely at the signal that sets off a muscle contraction (Fig. 9.15). When a neuron sends a signal to a muscle cell, it causes the muscle cell to shorten, and when many muscle cells shorten, the muscle as a whole contracts. The signaling molecule released from the neuron is a neurotransmitter called acetylcholine, which diffuses across the small space called the neuromuscular junction between the neuron and the muscle cell, until it encounters and binds to its receptor on the muscle cell. The acetylcholine receptor is a ligand-gated ion channel, so when acetylcholine binds to the extracellular ligand binding site, the conformational change that takes place opens the channel, and Na+ ions rush into the muscle cell. The sudden influx of Na+ ions changes the membrane potential, activating the contractile machinery of the cell and causing it to contract.
9-15
This sort of cell signaling needs a mechanism for shutting off just as the other receptor pathways do. In the case of ligand-gated channels, there are several places where regulation takes place. An important point of regulation is the length of time the ligand remains in the binding site of the receptor, since the occupation of the binding site causes the channel to remain open. A second point of regulation is provided by an enzyme called acetylcholinesterase, which is present in the neuromuscular junction and terminates the acetylcholine signal by rapidly breaking it down. When the binding site is unoccupied, the channel reverts to the closed conformation. The flow of Na+ ions into the cells ceases and the membrane potential returns to its resting state.
This signaling pathway is the target of a deadly poison known as curare. Native hunters of South America isolate curare from indigenous plants and rub it on arrowheads to kill their prey. Curare blocks the acetylcholine receptor, thereby relaxing voluntary muscles, including the diaphragm, which supports normal breathing. The result is death by asphyxiation. Interestingly, during the twentieth century, curare and its derivatives were used as a component of anesthesia to relax muscles during surgery. Today, anesthesiologists routinely use much safer and more effective blockers of the acetylcholine receptor to ease the manipulation of muscles and tissues during surgery.
9.4.4 How do cell signaling errors lead to cancer?
Case 2 Cancer: When Good Cells Go Bad
Many cancers arise when something goes wrong with the way a cell responds to a signal that leads to cell division or, in some cases, when a cell behaves as if it has received a signal for cell division when in fact it hasn’t. Problems with cell signaling that can lead to cancer can take place at just about every step in the cell-signaling process. In some rare cases, a tumor may form as the result of the overproduction of a signaling molecule or the production of an altered form of a signaling molecule. More frequently, the source of the problem is in the receptor. For example, individuals with some forms of cancer have from 10 to 100 times the normal number of receptors for a signaling molecule called epidermal growth factor (EGF), making the cell more responsive to the normally low levels of EGF in the environment. Under normal conditions, binding of EGF to its receptor leads to the controlled division of cells. But in these cancer patients, the heightened response of the signaling pathway leads to abnormal gene expression and excess cell division.
Individuals with other cancers, including certain breast cancers, have mutant EGF receptors that activate intracellular pathways even when EGF isn’t present. One of these mutant receptors, called Her2/neu, is the target of antibody therapy. Antibodies are proteins synthesized by cells of the immune system that target foreign substances in the body for destruction. Pharmaceutical companies are able to produce antibodies that specifically target the Her2/neu receptor. When these antibodies are used as a form of chemotherapy, they bind to the cancer cells with Her2/neu on their surfaces, targeting them for destruction by the immune system.
Farther down the pathway, mutant forms of the Ras protein are often present in cancers. One especially harmful mutation prevents Ras from converting its bound GTP to GDP. The protein remains locked in the active, GTP-bound state, causing the sustained activation of the MAP kinase pathway. More than 30% of all human cancers involve abnormal Ras activity as a result of one or more mutations in the ras gene.
9.4.5 Signaling pathways can intersect with one another in a cell.
In this chapter, we have focused on individual signaling pathways to emphasize the general principles of communication between cells. In each case, we saw that a cell releases or has a cell-surface signaling molecule, which binds to and activates a cell-surface or intracellular receptor. Binding of the signaling molecule to the receptor induces a conformational change in the receptor, causing it to become active. The activated receptor in turn causes changes in the interior of the cell, frequently turning on a signal-transduction cascade that is amplified, leading to a cellular response. Cellular responses can vary depending on the nature of the signal and type of cell. They include turning genes on or off, changes in cellular metabolism, and cell division. Eventually, the signal and response terminate.
Focusing on each pathway one at a time allowed us to understand how each pathway operates. But in the context of an organism, cell signaling can be quite complex. There are scores upon scores of different signaling molecules, most of which have their own specific receptor. The receptors may or may not be present on or in a particular cell, and if a certain receptor is present, the number of copies of that receptor may vary widely.
In addition, the cellular response to a particular signaling molecule can vary from one kind of cell to another. For example, in mammalian cells the MAP kinase pathway promotes cell division, whereas in yeast cells it triggers sexual reproduction. The various effects that activating the MAP kinase pathway can have on the cell depends in part on the different sets of proteins that are found in different cell types.
It is also important to realize that different signaling molecules can bind to a single cell and activate several signaling pathways simultaneously, so that the final response of a cell depends on how the pathways intersect with one another. The integration of different signals gives cells a wide range of possible responses to their environment. Receiving two different signals may enhance a particular response, such as cell growth; or one signal may inhibit a component of the signaling pathway triggered by the other signal, resulting in a weakened response. Thus, cells are able to use a similar set of signaling cascades to communicate with other cells and respond to the environment in myriad different ways.
9-16
For example, recent studies have shown that enzymes in the MAP kinase pathway can be inhibited by active PKA. Recall that PKA is activated by elevated cAMP levels associated with the G protein-coupled receptor pathway. The regulation of the MAP kinase pathway by PKA therefore illustrates how one signaling pathway can inhibit another.
Researchers have made use of this molecular cross talk. Many patients with breast cancer have elevated MAP kinase activity in their tumor cells. When human breast cancer cells are genetically modified to express an activated G protein α subunit, their growth after transplantation into mice is significantly inhibited. In addition, in cell culture, elevated cAMP levels block cells from responding to growth factors that signal through Ras to the MAP kinase pathway. As we understand more about how different signaling pathways integrate their outputs in specific cell types, we will stand a better chance of tailoring therapies to inhibit the pathway components relevant to a particular cancer.