10.2 THE CYTOSKELETON
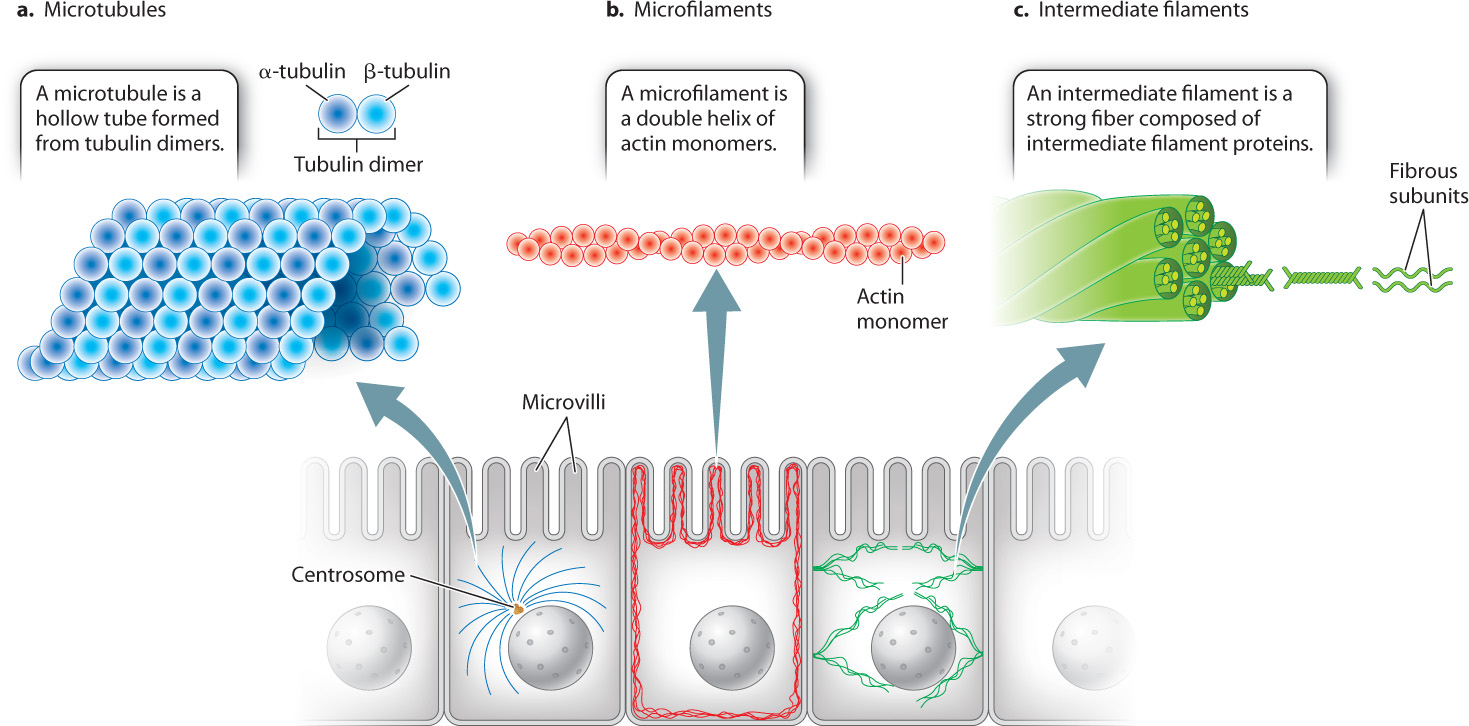
Just as the bones of vertebrate skeletons provide internal support for the body, the protein fibers of the cytoskeleton provide internal support for cells (Fig. 10.3). All eukaryotic cells have at least two cytoskeletal elements, microtubules and microfilaments. Animal cells have a third element, intermediate filaments. All three of these cytoskeletal elements are long chains, or polymers, made up of protein subunits. In addition to providing structural support, microtubules and microfilaments enable the movement of many cells and the movement of substances within cells. We first consider the structural role of the cytoskeleton and return to its role in cell motility and intracellular transport in section 10.3.
10-4
10.2.1 Microtubules are hollow, tubelike polymers of tubulin dimers.
Microtubules, which are hollow tubelike structures, have the largest diameter of the three cytoskeletal elements (Fig. 10.3a). They are polymers of protein dimers. Each protein dimer is made up of two slightly different tubulin proteins, called α (alpha) and β (beta) tubulin. One α tubulin and one β tubulin combine to make a tubulin dimer. These tubulin dimers are assembled into microtubules.
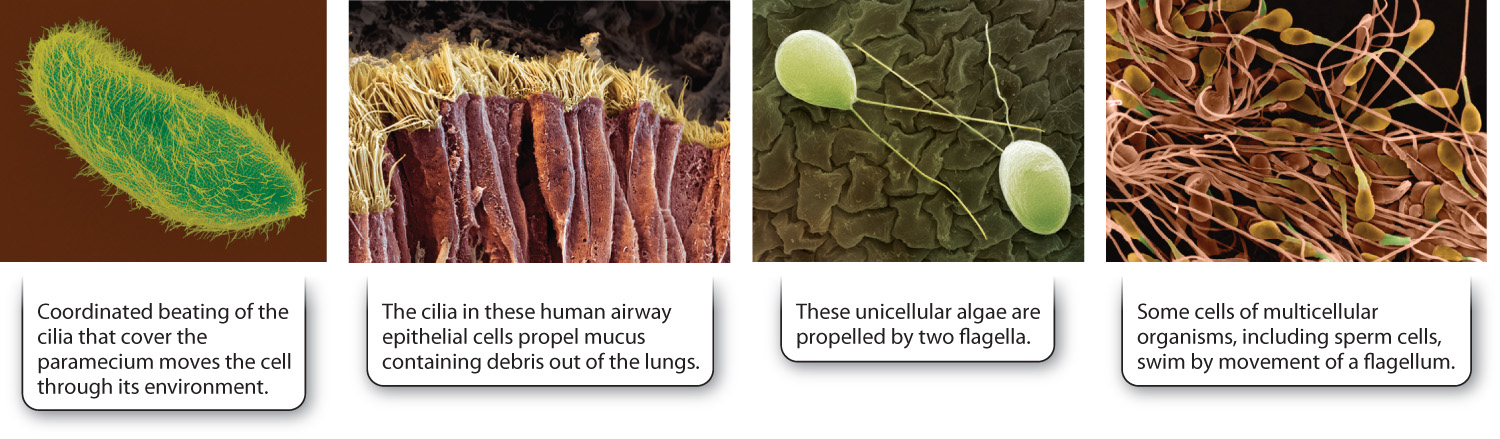
Microtubules have diverse functions. In animal cells, microtubules radiate outward to the cell periphery from a microtubule organizing center called the centrosome. This spokelike arrangement of microtubules helps cells withstand compression and thereby maintain their shape. Many organelles are tethered to microtubules, which guide the arrangement of organelles in the cell. Microtubules also provide tracks for the transport of material from one part of the cell to another. They are found specially arranged in cilia and flagella, organelles that propel the movement of cells or substances surrounding the cell (Fig. 10.4). Finally, microtubules form the spindle apparatus that separates replicated chromosomes during eukaryotic cell division (Chapter 11).
10.2.2 Microfilaments are helical polymers of actin.
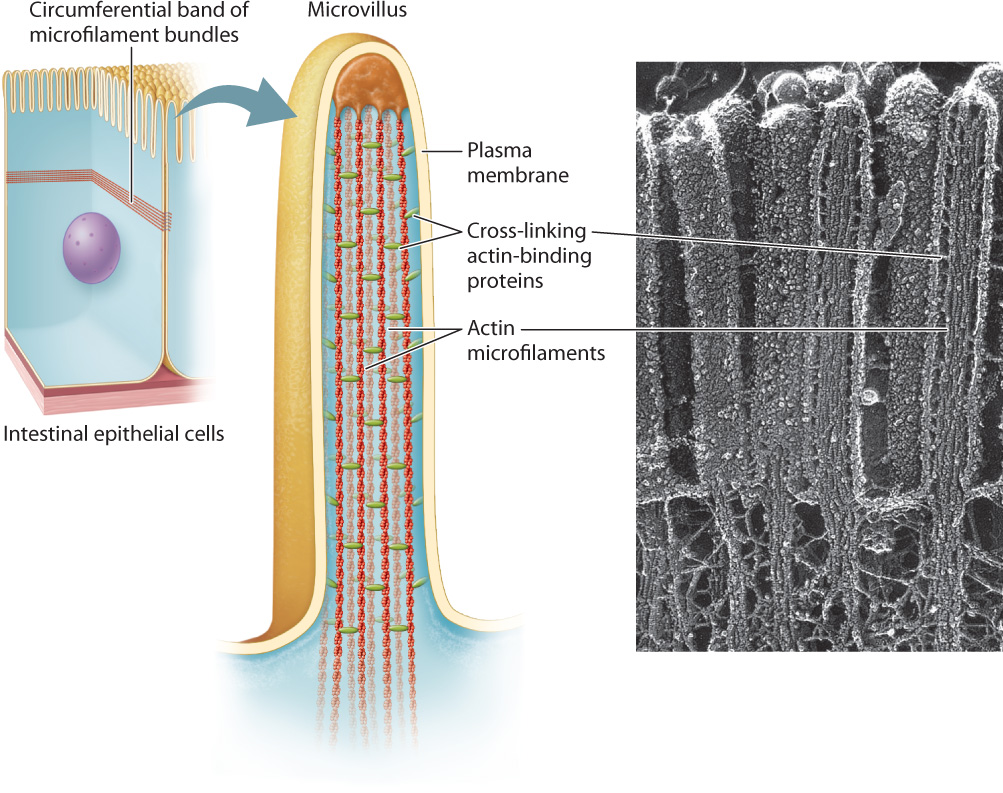
Microfilaments are polymers of actin monomers, arranged to form a helix. They are the thinnest of the three cytoskeletal fibers and are present in various locations in the cytoplasm (see Fig. 10.3b). They are relatively short and extensively branched in the cell cortex, the area of the cytoplasm just beneath the plasma membrane. At the cortex, microfilaments reinforce the plasma membrane and organize proteins associated with it. These cortical microfilaments are also important in maintaining the shape of a cell. For example, in absorptive epithelial cells such as those in the small intestine, bundles of microfilaments are found in microvilli, hairlike projections that extend from the surface of the cell (Fig. 10.5). Longer bundles of microfilaments form a band that extends around the circumference of epithelial cells. This band is attached to a type of cell junction called an adherens junction, which connects a cell to its neighbors (section 10.4). As a result, the band provides a great deal of structural support not only to individual epithelial cells, but also to the entire epithelial layer of cells.
In addition to providing structural support to the cell, microfilaments are important for the transport of materials inside cells, especially plant cells. Furthermore, microfilaments are responsible for changes in the shape of many types of cells. An exceptionally dramatic example of such a shape change is the shortening of a muscle cell when it contracts, discussed more fully in the next section. Microfilaments are found at the front edge of migrating cells, where they can move the plasma membrane forward. Finally, microfilaments, arranged in a structure called the contractile ring separate the daughter cells at the end of animal cell division (Chapter 11).
10.2.3 Intermediate filaments are polymers of proteins that vary according to cell type.
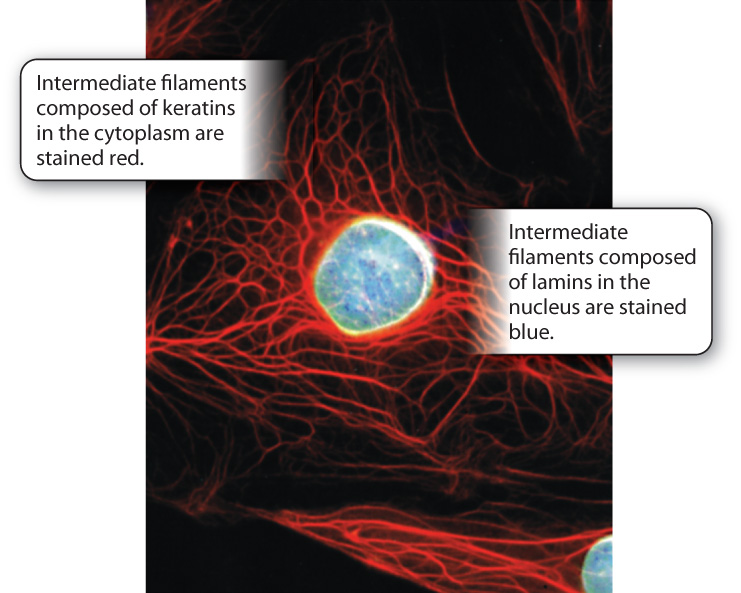
The intermediate filaments of animal cells are so named because their diameter is intermediate between that of microtubules and microfilaments (see Fig. 10.3c). They are polymers of intermediate filament proteins that combine to form strong, cable-like structures in the cell. As a result, they provide cells with mechanical strength. We have seen that different cell types all use the same tubulin dimers to form microtubules and the same actin monomers to form microfilaments. By contrast, the proteins making up intermediate filaments differ from one cell type to another. For example, in epithelial cells, these protein subunits are keratins; in fibroblasts, they are vimentins; and in neurons, they are neurofilaments. Some intermediate filaments, called lamins, are even found inside the nucleus, where they provide support for the nuclear envelope (Fig. 10.6). In fact, there are well over 100 different kinds of intermediate filaments.
10-5
Once assembled, many intermediate filaments become attached at the cytoplasmic side of cellular junctions called desmosomes (Fig. 10.7; section 10.4). Like the association of microfilaments with adherens junctions, the anchoring of intermediate filaments to desmosomes provides strong support for the cells. In the case of epithelial cells, this anchoring results in structural continuity from one cell to another that greatly strengthens the entire epithelial tissue, enhancing the ability of epithelial cell layers to withstand physical stress. This is especially important in a wide range of tissues that are regularly subject to such stress, including the skin and the lining of the intestine.
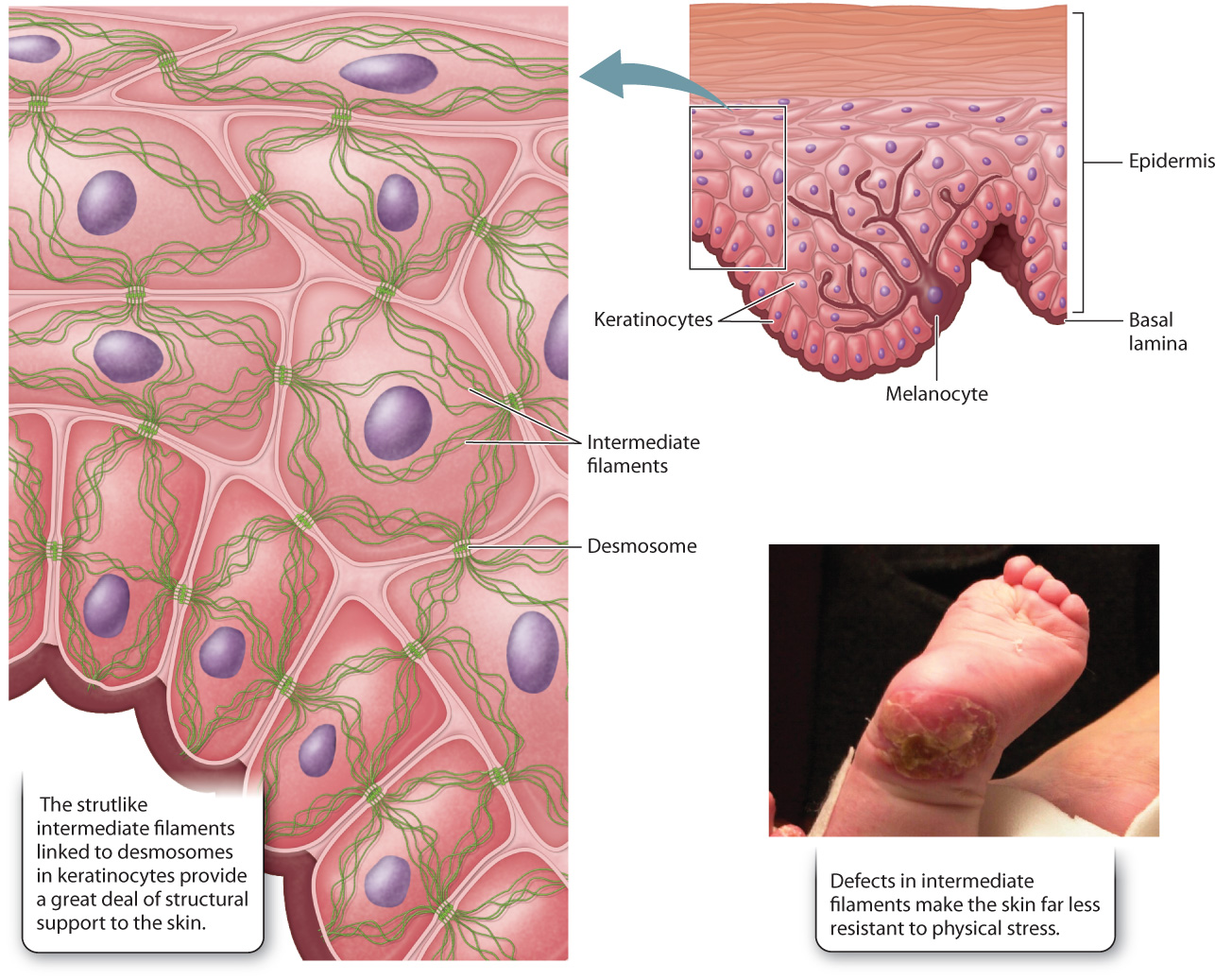
Genetic defects that disrupt the intermediate filament network can have severe consequences. For example, some individuals with epidermolysis bullosa, a group of rare genetic diseases, have defective keratin genes inherited from their parents. Intermediate filaments do not polymerize properly in these individuals, weakening connections between the layers of cells that make up the epidermis. As a consequence, the outer layers can detach, resulting in extremely fragile skin that blisters in response to the slightest trauma (Fig. 10.7). The sensitivity to physical stress is so extreme that infants with epidermolysis bullosa often suffer significant damage to the skin during childbirth. Therefore, caesarean section is sometimes recommended in cases where the disease is diagnosed during pregnancy.
10.2.4 How can doctors test for the spread of cancer?
Case 2 Cancer: When Good Cells Go Bad
Cancer is characterized by uncontrolled cell division. Many types of cancer result in the formation of a mass of cells called a tumor. In most cases, the tumor itself is not the problem. The real concern is that tumor cells will metastasize, or spread to other parts of the body, where they can affect the function of distant organs. One way physicians check for metastasis is to look for intermediate filament proteins in places where they don’t belong. For example, the presence of certain keratins in the bloodstream is an indicator of metastasis.
Physicians may also examine lymph nodes for signs that a cancer has spread. Lymph nodes are part of the lymphatic system, which collects extracellular fluid from tissues in the body and returns it to the circulatory system. Each part of the body drains into a specific set of lymph nodes. So if a patient is diagnosed with a melanoma, a cancer of melanocytes, a surgeon can remove the lymph node that drains the area of the body where the cancer is. A pathologist then examines the cells of the lymph node for the presence of melanocyte keratins. Since the cells of the lymph node do not normally contain melanocyte keratins, the presence of these intermediate filament proteins in the lymph node helps doctors determine how advanced the cancer is and thus design the best course of treatment. This lymph node test is useful in detecting other kinds of cancer as well, including cancers of the breast and prostate.
10-6
10.2.5 Microtubules and microfilaments are dynamic structures.
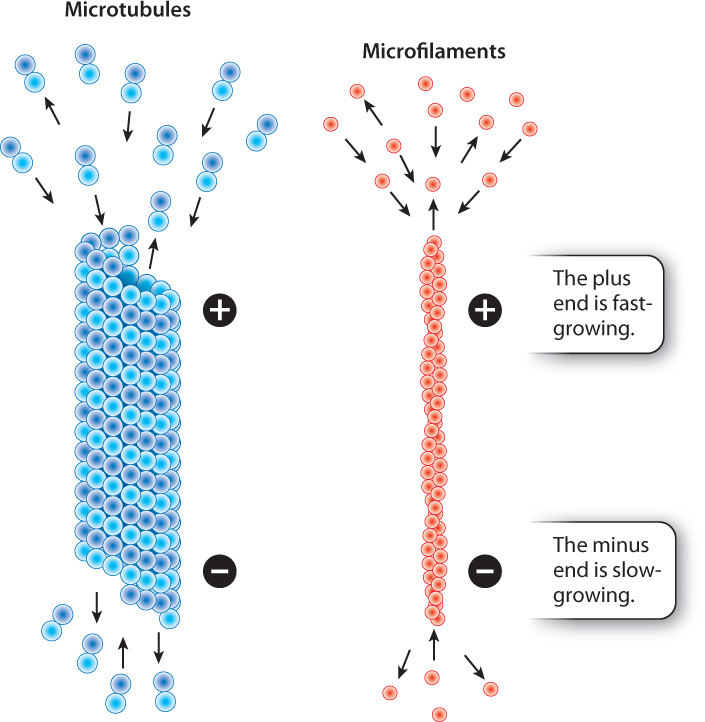
When we think of the parts of a skeleton, we are inclined to think of our bones. Unlike our bones, which undergo remodeling but do not change rapidly, microtubules and microfilaments are highly dynamic structures. They become longer by the addition of subunits to their ends, and shrink by the loss of subunits. The growth and shrinkage of microtubules and microfilaments are influenced by many factors, including the concentration of free tubulin and actin subunits and the activity of regulatory proteins that attach to the cytoskeleton.
The rate at which protein subunits are added depends on the concentrations of tubulin and actin in that region of the cell. At high concentrations of subunits, microtubules and microfilaments may grow at both ends, although these subunits are added more quickly to one end than to the other. The faster-growing end is called the plus end and the slower-growing end is called the minus end (Fig. 10.8). The minus ends of microtubules in animal cells are positioned at the organizing center of the centrosome, and the plus ends project outward toward the plasma membrane.
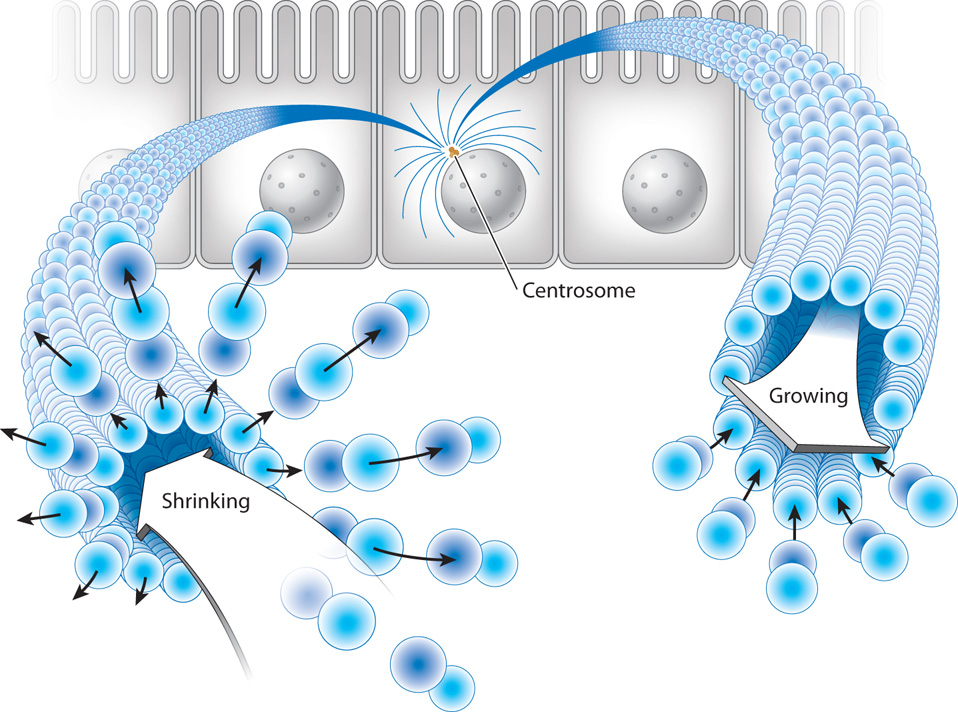
In addition, microtubules have an important property not shared by the other cytoskeletal elements: Their plus ends undergo seemingly random cycles of rapid shrinkage (depolymerization) followed by slower growth (polymerization). These cycles of shrinkage and growth are called dynamic instability (Fig. 10.9). The dramatic shrinkage is often called microtubule catastrophe and takes place because the plus end of a microtubule is structurally unstable. Once depolymerization has occurred, tubulin dimers are added to reassemble the microtubule.
10-7
Dynamic instability might seem like an undesirable feature for a component of the cytoskeleton, but it is actually very important for many functions of microtubules. For example, it allows microtubules to explore the space of the cell by growing into new areas and then shrinking back. This ability is especially important in the process of dividing chromosomes between the two daughter cells during cell division. In Chapter 11, we will see that the segregation of chromosomes requires that each chromosome be attached to two microtubules. The way that microtubules encounter chromosomes fast enough to form these attachments is by random exploration of the cytoplasm, which is driven by dynamic instability.
10.2.6 The cytoskeleton is an ancient feature of cells.
Actin and tubulin are found in all eukaryotic cells, and evolutionarily they are highly conserved. The amino acid sequences of yeast tubulin and human tubulin are 75% identical. Similar comparisons of actin from amoebas and animals show that they are 80% identical after close to a billion years of evolution. In fact, a mixture of yeast and human actin monomers forms hybrid microfilaments able to function normally in the cell.
Not long ago, it was believed that cytoskeletal proteins were present only in eukaryotic cells. However, a number of studies have shown that many prokaryotes also have a system of proteins similar in structure to the cytoskeletal elements of eukaryotic cells and are involved in similar processes, including the separation of daughter cells during cell division. Interestingly, at least one of these prokaryotic cytoskeleton-like proteins is expressed in chloroplasts and mitochondria of some eukaryotic cells. The presence of this protein in these organelles lends support to the theory that chloroplasts and mitochondria were once independent prokaryotic cells that developed a symbiotic relationship with ancestral eukaryotic cells. This idea is called the endosymbiotic theory and we discuss it in more detail in Chapter 27.
10-8