11.4 REGULATION OF THE CELL CYCLE
Both mitotic and meiotic cell division must occur only at certain times and places. Mitotic cell division, for example, occurs during growth of a multicellular organism, wound healing, or in the maintenance of actively dividing tissues such as the skin or lining of the intestine. Similarly, meiotic cell division occurs only at certain times during development. Cell division cannot occur all the time because uncontrolled cell proliferation is dangerous—in fact, it is a hallmark of cancer. Even for unicellular organisms, cell division needs to be regulated so that it takes place only when conditions are favorable—for example, when enough nutrients are present in the environment.
Cells do not proceed to cell division until they are ready. Has all of the DNA been accurately replicated during S phase? Has the cell grown to a sufficient size to support division into viable daughter cells? If these and other preparations have not been accomplished, the cell halts its progression through the cell cycle. The cell has regulatory mechanisms for spotting faulty or incomplete preparations and arresting cell division. When these mechanisms fail, the resulting daughter cells are abnormal, and either may not survive or may run the risk of dividing inappropriately. In this section, we consider how cells control their passage through the cell cycle. Our focus is on control of mitotic cell division. However, many of the same factors also regulate meiotic cell division, a reminder of the close evolutionary connection between these two forms of cell division.
11.4.1 Protein phosphorylation controls passage through the cell cycle.
Early animal embryos, such as those of frogs and sea urchins, are useful models for studying cell cycle control because they are large and undergo many rapid mitotic cell divisions following fertilization. During these rapid cell divisions, mitosis and S phase alternate with virtually no G1 and G2 phases in between. Studies of animal embryos revealed two interesting patterns. First, as the cells undergo this rapid series of divisions, several proteins appear and disappear in a cyclical fashion. Researchers interpreted this observation to mean that they might play a role in the control of the progression through the cell cycle. Second, several enzymes become active and inactive in cycles. These enzymes are kinases, proteins that phosphorylate other proteins (Chapter 9). The timing of kinase activity is delayed slightly relative to the appearance of the cyclical proteins.
11-15
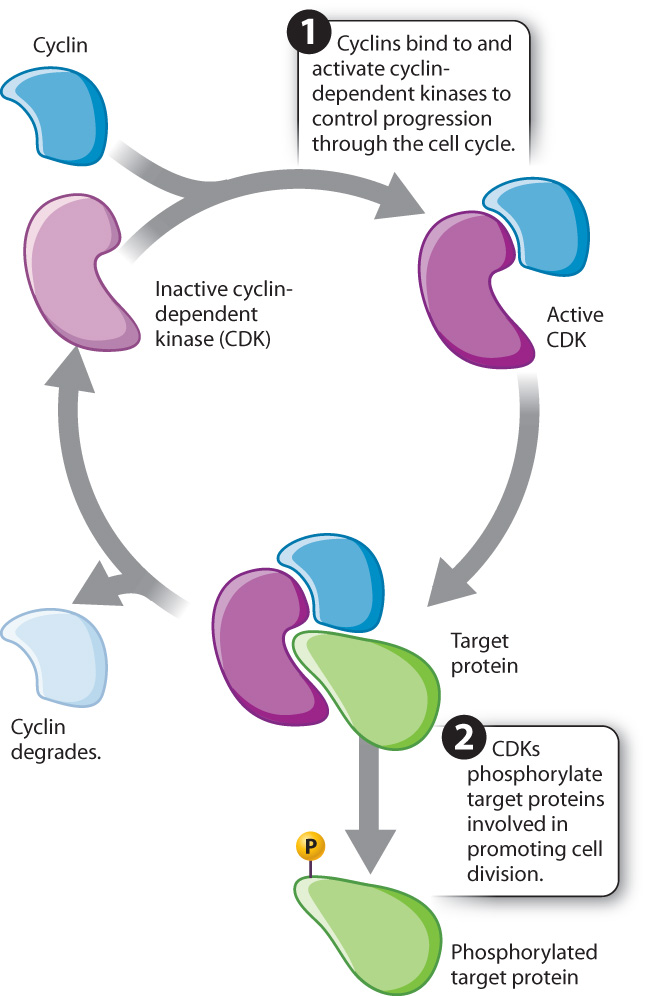
These and many other observations led to the following view of cell cycle control: Proteins are synthesized that activate the kinases. These regulatory proteins are called cyclins because their levels rise and fall with each turn of the cell cycle. Once activated by cyclins, the kinases phosphorylate target proteins involved in promoting cell division (Fig. 11.14). These kinases, cyclin-dependent kinases, or CDKs, are always present within the cell but are active only when bound to the appropriate cyclin. It is the kinase activity of the cyclin–CDK complexes that triggers the required cell cycle events. Therefore, the cyclical change in cyclin–CDK activity depends on the cyclical levels of the cyclins.
These cell cycle control proteins, including cyclins and CDKs, are widely conserved across eukaryotes, reflecting their fundamental role in controlling cell cycle progression, and have been extensively studied in yeast, sea urchins, mice, and humans (Fig. 11.15).
FIG. 11.15: How is progression through the cell cycle controlled?
BACKGROUND The cell cycle is characterized by cyclical changes in many components of the cell: Chromosomes condense and decondense, the mitotic spindle forms and breaks down, the nuclear envelope breaks down and re-forms. How these regular and cyclical changes are controlled is the subject of active research. First, clues came from studies in yeast in which mutations in certain genes blocked progression through the cell cycle, suggesting that these genes encode proteins that play a role in cell cycle progression. Additional clues came from studies in embryos of the sea urchin Arbacia punctulata. These embryos are large and divide rapidly by mitosis, making them a good model system for the study of the control of cell division. In the early 1980s, it was known that inhibition of protein synthesis blocks key steps of cell division in sea urchins. It was also known that an enzyme called MPF, or M-phase promotion factor, is important for the transition from G2 to M phase.

EXPERIMENT To better understand events in the cell cycle, English biochemist Tim Hunt and colleagues measured protein levels in sea urchin embryos as they divide rapidly by mitosis. He added radioactive methionine (an amino acid) to eggs, which became incorporated into any newly synthesized proteins. The eggs were then fertilized and allowed to develop. Samples of the rapidly dividing embryos were taken every 10 minutes and run on a gel to visualize the levels of different proteins.
RESULTS Most protein bands became darker as cell division proceeded, indicating more and more protein synthesis. However, the level of one protein band oscillated, increasing in intensity and then decreasing with each cell cycle, as shown in the graph.
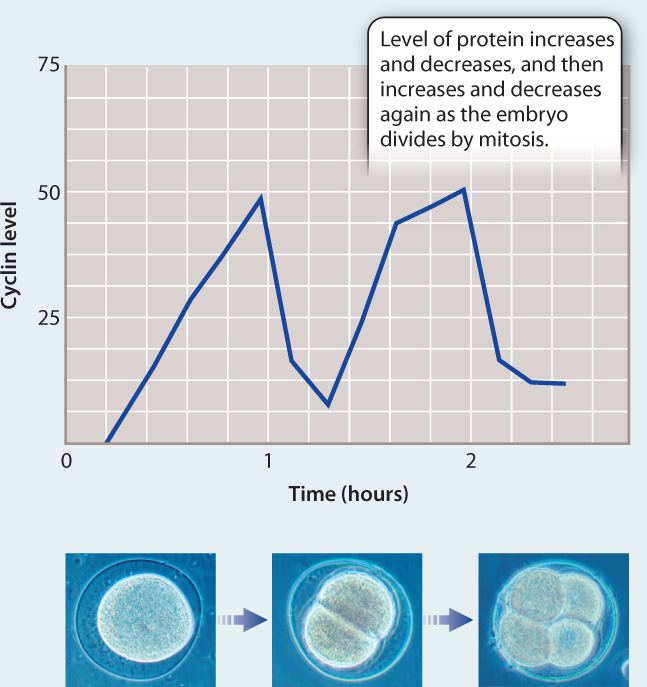
CONCLUSION Hunt and colleagues called this new protein “cyclin.” Although they did not know its function, its fluctuating level suggested it might play a role in the control of the cell cycle. However, more work was needed to figure out if cyclins actually cause progression through the cell cycle or whether their levels oscillate in response to progression through the cell cycle.
FOLLOW-UP WORK Hunt and colleagues found a similar rise and fall in the levels of certain proteins in the sea urchin Lytechinus pictus and surf clam Spisula solidissima. MPF was found to consist of a cyclin protein and a cyclin-dependent kinase that play a key role in the G2–M transition. In other words, cyclins do in fact control progression through the cell cycle by their interactions with cyclin-dependent kinases. Hunt shared the Nobel Prize in Physiology or Medicine in 2001 for his work on cyclins.
SOURCE T. Evans, E. T. Rosenthal, J. Youngblom, D. Distel, and T. Hunt. 1983. “Cyclin: A Protein Specified by Maternal mRNA in Sea Urchin Eggs That Is Destroyed at Each Cleavage Division.” Cell 33:389–396.
11-16
11.4.2 Different cyclin–CDK complexes regulate each stage of the cell cycle.
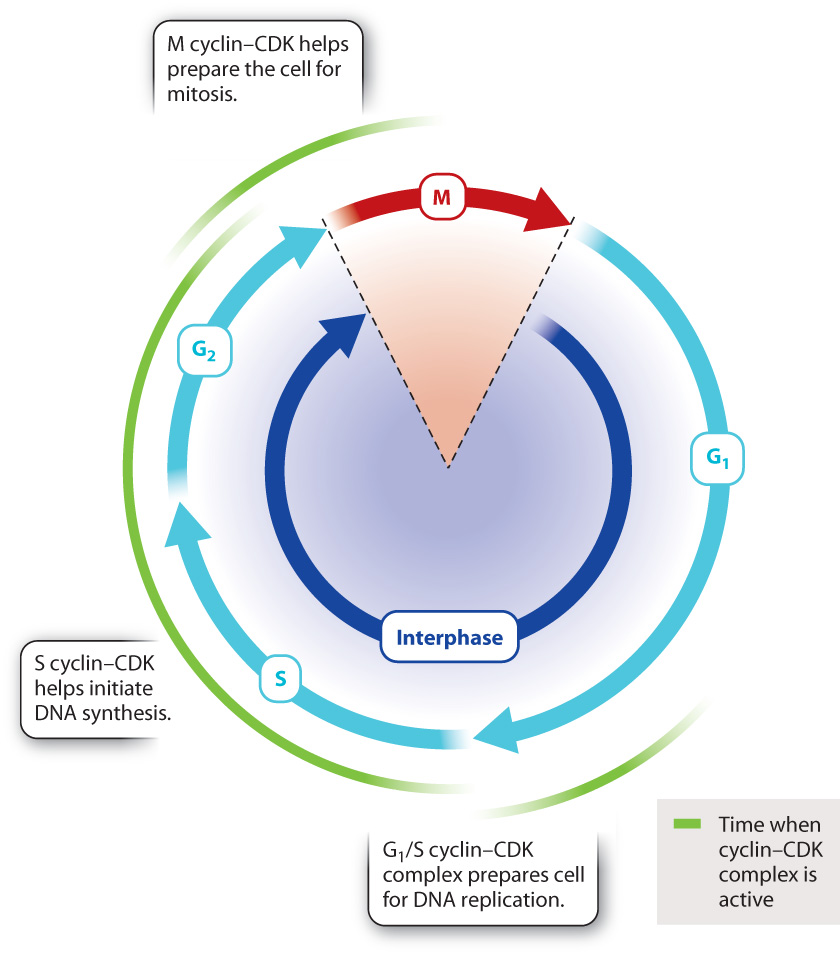
In mammals, there are several different cyclins and CDKs that act at specific steps of the cell cycle. Three steps in particular are subject to cyclin–CDK regulation in all eukaryotes (Fig. 11.16).
The G1/S cyclin–CDK complex, which is active at the end of the G1 phase, is necessary for the cell to enter S phase. For example, this cyclin–CDK complex activates a protein that promotes the expression of histone proteins needed for packaging the newly replicated DNA strands.
The S cyclin–CDK complex is involved in the initiation of DNA synthesis. It activates protein complexes involved in DNA replication that contain enzymes necessary for DNA synthesis. Once replication has begun at a particular place on the DNA, S cyclin–CDK activity prevents the replication complex from reassembling at the same place and re-replicating the same DNA sequence. Synthesizing the same sequence repeatedly would be dangerous because cells with too much or not enough DNA could die or become cancerous.
The M cyclin–CDK complex initiates multiple events associated with mitosis. For example, M cyclin–CDK phosphorylates structural proteins in the nucleus, triggering the breakdown of the nuclear envelope in prophase. M cyclin–CDK also phosphorylates proteins that regulate the assembly of tubulin into microtubules, promoting the formation of the mitotic spindle.
11.4.3 Cell cycle progression requires successful passage through multiple checkpoints.
The regulatory system represented by cyclins and CDKs gives the cell opportunities to halt the cell cycle should something go wrong. In other words, if the preparations for the next stage of the cell cycle are incomplete or if there is some kind of damage, regulatory mechanisms block the cyclin–CDK activity required for the next step, pausing cell division until preparations are complete or the damage is repaired. Each regulatory mechanism is called a checkpoint.
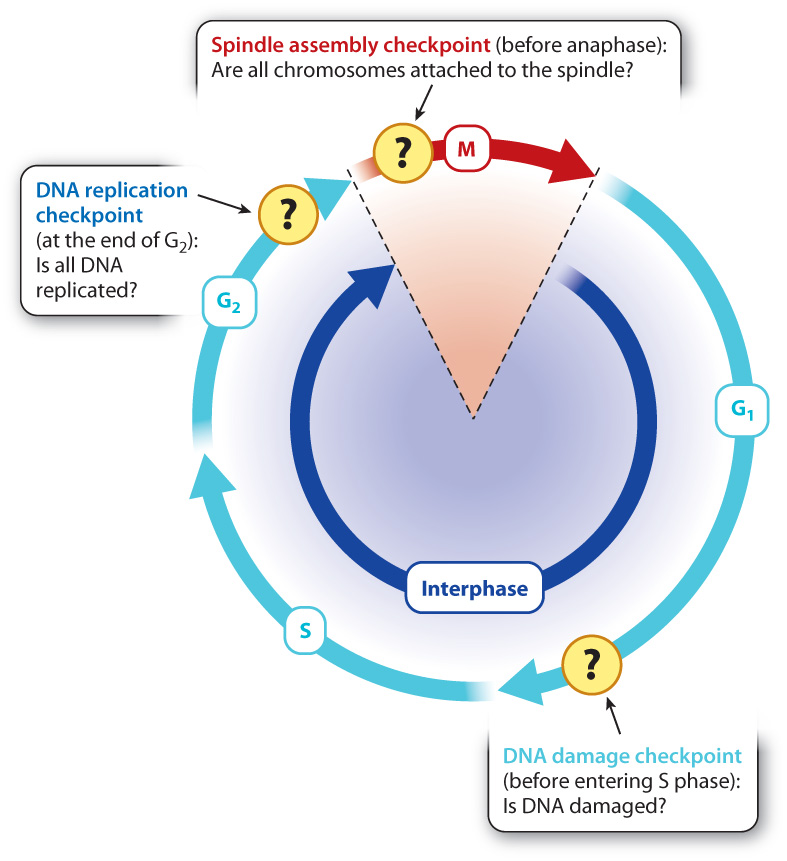
Cells have many cell cycle checkpoints. Three major checkpoints that have been well studied are illustrated in Fig. 11.17. The presence of unreplicated DNA arrests the cell at the end G2 before the cell enters mitosis, and abnormalities in chromosome attachment to the spindle arrest the cell in early mitosis. By way of illustration, we focus on a key checkpoint that responds to the presence of damaged DNA that occurs at the end of G1.
DNA can be damaged by environmental insults such as ultraviolet radiation or chemical agents. Typically, damage takes the form of double-stranded breaks in the DNA. If the cell progresses through mitosis with DNA damage, the damage might be inherited by the daughter cells or the chromosomes might not segregate normally. Some checkpoints delay progression through the cell cycle until DNA damage is repaired. An important one occurs in late G1. This DNA damage checkpoint depends on several regulatory proteins, some of which recognize damaged DNA while others arrest cell cycle progression before S phase (Fig. 11.17).
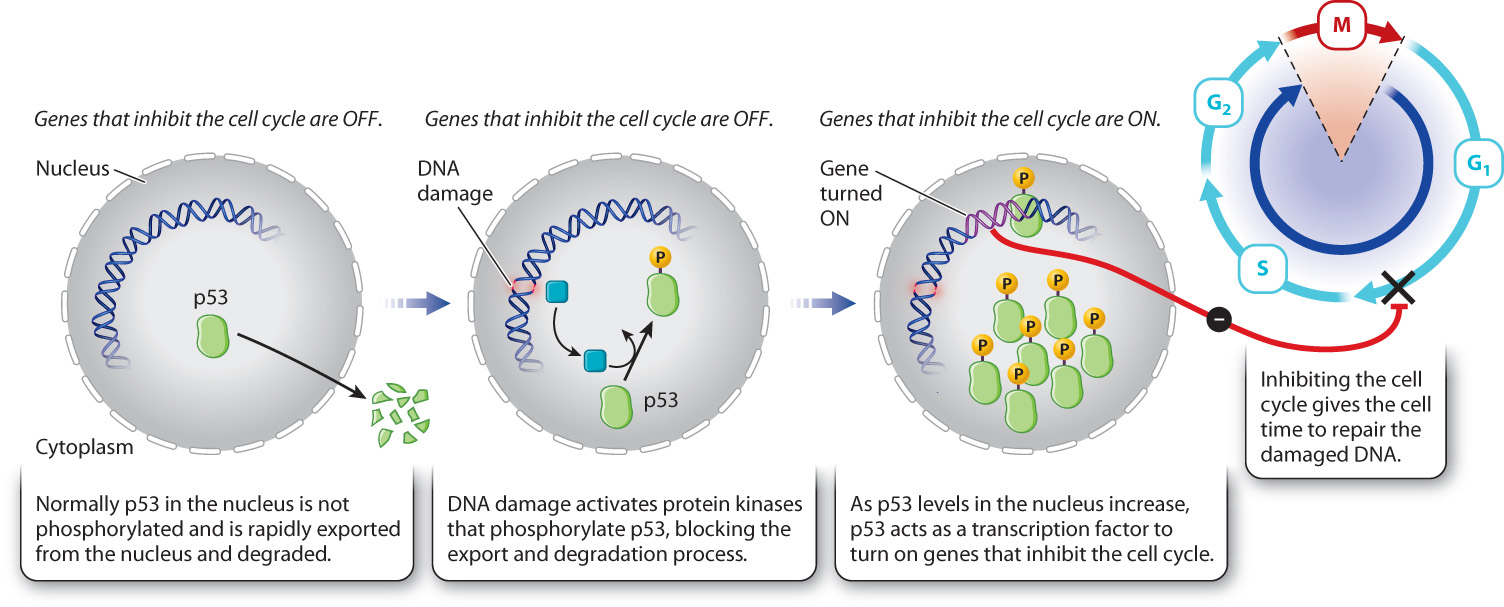
When DNA is damaged by radiation, a specific protein kinase is activated that phosphorylates a protein called p53. Normally, p53 is exported from the nucleus and therefore is at very low levels in the nucleus. Phosphorylation of p53 prevents its export and thereby increases its levels in the nucleus. As p53 levels rise, p53 activates the transcription of a gene that expresses a CDK inhibitor protein. This inhibitor binds to and blocks the activity of the G1/S cyclin–CDK complex (Fig. 11.18). In so doing, p53 arrests the cell at the G1/S transition, giving the cell time to repair the damaged DNA.
Question Quick Check 7
bp9fq37HSf9cFgB9l9b24E85RP7OzjYNp/KphfGT0h16pJlXVdGD5pPN2/ab7GUibZssgVnVAU3f4m37HsVOY1rNVES/STyD+a9p2w==11-17