12.4 RECOMBINANT DNA AND GENETICALLY MODIFIED ORGANISMS
Along with methods to manipulate DNA fragments came the capability of isolating genes from one species and introducing them into another. This type of genetic engineering is called recombinant DNA technology because it literally recombines DNA molecules from two (or more) different sources into a single molecule. Recombinant DNA technology involves cutting DNA by restriction enzymes, isolating them by gel electrophoresis, and ligating them with enzymes used in DNA replication. This technology is possible because the DNA of all organisms is the same, differing only in sequence but not in chemical or physical structure. When DNA fragments from two different sources are combined into a single molecule and incorporated into a cell, they are replicated and transcribed just like any other DNA molecule.
Recombinant DNA technology can combine DNA from any two sources, including two different species. DNA from one species of bacteria can be combined with another, or a human gene can be combined with bacterial DNA, or the DNA from a plant and a fungus can be combined into a single molecule. These new sequences may be unlike any found in nature, raising questions about their possible effects on human health and the environment.
12-18
This section discusses one of the basic methods for producing recombinant DNA and also some of the important (and controversial) applications of recombinant DNA technology, such as genetically modified food.
12.4.1 Recombinant DNA combines DNA molecules from two sources.
The first application of recombinant DNA technology was the introduction of foreign DNA fragments into the cells of bacteria in the early 1970s. The method is simple and straightforward, and remains one of the mainstays of modern molecular research. It can be used to generate a large quantity of a protein for study or therapeutic use.
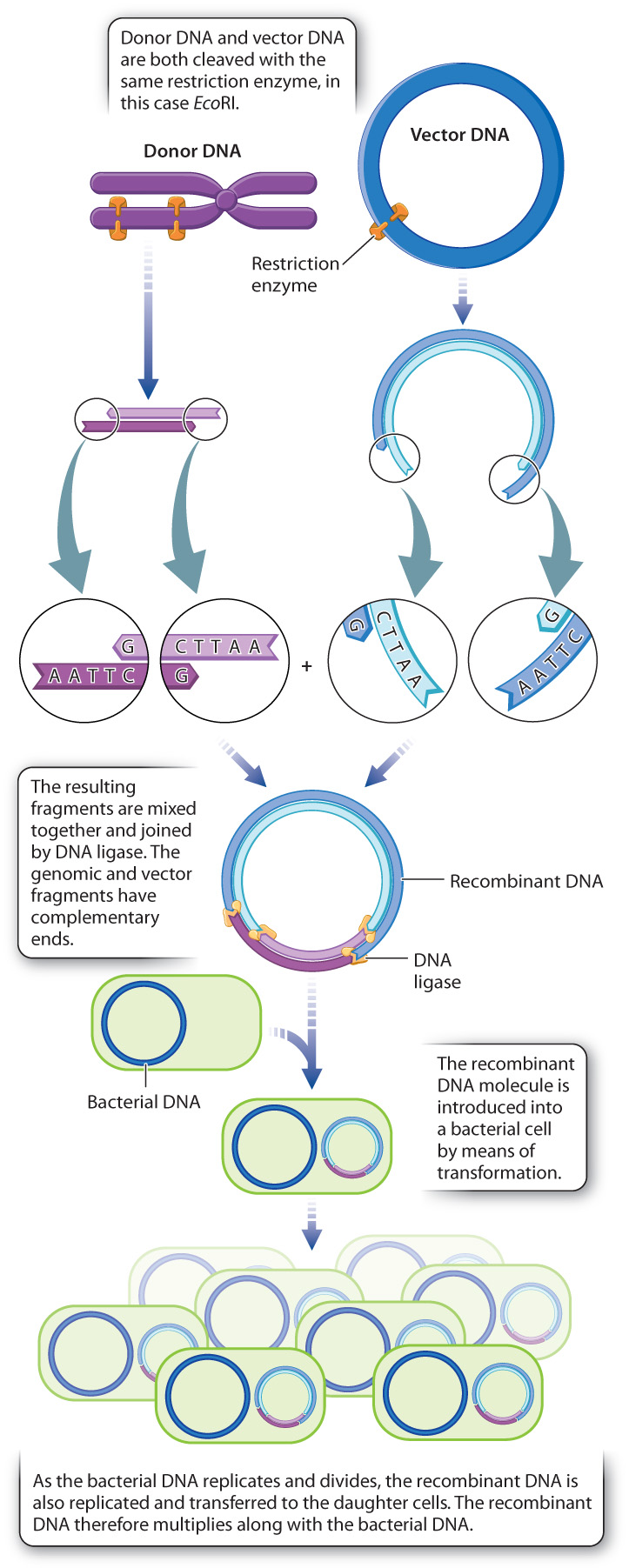
The method requires a fragment of double-stranded DNA that serves as the donor. The donor fragment may be a protein-coding gene, a regulatory part of a gene, or any DNA segment of interest. If you were interested in generating bacteria that could produce human insulin, you might use the coding region of the human insulin gene as your donor DNA molecule. Also required is a vector sequence into which the donor fragment is to be inserted. The vector is the carrier of the donor fragment, and it must have the ability to be maintained in bacterial cells. A frequently used vector is a bacterial plasmid, a small circular molecule of DNA found naturally in certain bacteria that can replicate when the bacterial genomic DNA replicates and be transmitted to the daughter bacterial cells when the parental cell divides. Many naturally occurring plasmids have been modified by genetic engineering to make them suitable for use as vectors in recombinant DNA technology.
A common method for producing recombinant DNA is shown in Fig. 12.19. In order to make sure donor DNA can be fused with vector DNA, both pieces are cut with the same restriction enzyme so they will both have the same overhangs. In the example shown in Fig. 12.19, the donor DNA is a fragment produced by digestion of genomic DNA with the restriction enzyme EcoRI, resulting in four-nucleotide 5′ overhangs at the fragment ends. The vector is a circular plasmid that contains a single EcoRI cleavage site, so digestion of the vector with EcoRI opens the circle with a single cut that also has four-nucleotide 5′ overhangs. Note that the overhangs on the donor fragment and the vector are complementary in sequence, which allows the ends of the donor fragment to renature with the ends of the opened vector when the two types of molecule are mixed. Once this renaturation has taken place, the ends of the donor fragment and the vector are covalently joined by DNA ligase. The joining of the donor DNA to the vector creates the recombinant DNA molecule.
The next step in the procedure is transformation, in which the recombinant DNA is mixed with bacteria that have been chemically coaxed into a physiological state in which they take up DNA from outside the cell. Having taken up the recombinant DNA, the bacterial cells are transferred into growth medium, where they multiply. Since the vector part of the recombinant DNA molecule contains all the DNA sequences needed for its replication and partition into the daughter cells, the recombinant DNA multiplies as the bacterial cell multiplies. If the recombinant DNA functions inside the bacterial cell, then new genetic characteristics may be expressed by the bacteria. For example, the recombinant DNA may allow the bacterial cells to produce a human protein, such as insulin or growth hormone.
12-19
Question Quick Check 4
izTVyGQxiEPPosGuFbD9QmNIgwLLmqRlaFNGIoyCpbmarLvBAgumzhwnmAKRyPS2jphT+enfbEyOxfUSzZgx77ngnnv7Fe1DIjAgoqGrUW2Z6ioG5MeWiaY/pYqRwTj1H+UWY/IoyxHflDdk2nmKZfOpPPN02jkXUa5S++E6wt6pVteR+35WD47Wc7lIFnL46KvBQq9sE82In9iY7XqzAzKkvvmTmWNelmY6qa+8NdLAAwuVYHx2omiY+hoL/c6Nwo2OG1H3CvXBpZGj8xhfZMfP6mTxvJhjDR/jhZF2oGdEeqWw0OssQ2xf9ovAHFR+vZPj68bDVw1jTJXe/fECAObe5+wZ/t6sCsJHCOq7F+xHG9KfzeJA4KZGW8XL44ryamTTCXiGNBgKbZxm6pmt8QcQDPSZqJWXZOpnFM/6V91RpO+NE/RV0Nl0nUACLSLXzQ0S/BU5bM7QsIy3jDnrZb851dZBR6Pq4tRctmj1cpbj2+7DEfvlJKrfte83tRlWdljgM0VIUibAvIfPdAavNxJEE/OP4SIKm3X2/SEgnDgO3w9kF+MwMqX8d0Hv0dAiugdj3FSm1YCE7YgS12.4.2 Recombinant DNA is the basis of genetically modified organisms.

Applications of recombinant DNA have gone far beyond genetically engineered bacteria. Using methods that are conceptually similar to those described for bacteria but differing in many details, scientists have been able to produce varieties of genetically engineered viruses and bacteria, laboratory organisms, agricultural crops, and domesticated animals (Fig. 12.20). Examples include sheep that produce a human protein in their milk used to treat emphysema, chickens that produce eggs containing human antibodies to help fight bacteria, and salmon with increased growth hormone for rapid growth. Plants such as corn, canola, cotton, and many others have been engineered to resist insect pests, and other engineered products are rice with a high content of vitamin A, tomatoes with delayed fruit softening, potatoes with waxy starch, and sugarcane with increased sugar content. To model disease, researchers have used recombinant DNA to produce organisms such as laboratory mice that have been engineered to develop heart disease and diabetes. By studying these organisms, researchers can better understand human diseases and begin to find new treatments for them.
12-20
Genetically engineered organisms are known as transgenic organisms or genetically modified organisms (GMOs). Transgenic laboratory organisms are indispensable in the study of gene function and regulation and to identify genetic risk factors for disease. In crop plants and domesticated animals, GMOs promise enhanced resistance to disease, faster growth and higher yields, more efficient utilization of fertilizer or nutrients, and improved taste and quality. But there are concerns about unexpected effects on human health or the environment, the increasing power and influence of agribusiness conglomerates, and ethical objections to tampering with the genetic makeup of animals and plants. Nevertheless, more than 250 million acres of GMO crops are grown annually in more than 20 countries. The majority of this acreage is in the United States and South America. Resistance to the use of GMOs in Europe remains strong and vocal.