16.5 PATTERNS OF INHERITANCE OBSERVED IN FAMILY HISTORIES
Segregation of alleles takes place in human meiosis just as it does in peas and most other sexual organisms. The results are not so easily observed as in peas, however, for several reasons. First, humans do not choose their mating partners for the convenience of biologists, and so experimental crosses are not possible. Second, the number of children in human families is relatively small, and so the Mendelian ratios are often obscured by random fluctuations due to chance. Nevertheless, in many cases the occurrence of segregation can be observed even in human families. The patterns are often easiest to spot for inherited traits that are rare because in those cases the mutant alleles occur only in affected individuals and their close relatives.
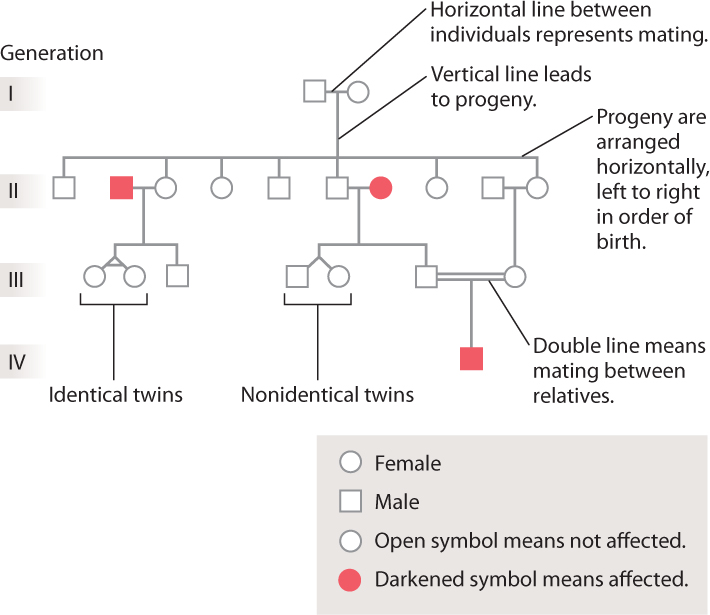
In studying human families, the record of the ancestral relationships among individuals is summarized in a diagram of family history called a pedigree. Some typical symbols used in pedigrees are shown in Fig. 16.17. Females are represented as circles and males as squares. If the pedigree is following the transmission of a disease from one generation to the next, unaffected individuals are indicated by open symbols and affected individuals by darkened symbols. A mating is represented as a horizontal line connecting the partners, and the children of the partners are arranged below them, left to right in order of birth. The same patterns of dominance and recessiveness that Mendel observed in his pea plants can be seen in some pedigrees. Over the next few sections, we’ll see how to recognize telltale features of a pedigree that reveal the genotypic nature of a trait.
16-15
16.5.1 Dominant traits appear in every generation.
The pedigree shown in Fig. 16.18 is for a rare dominant trait, brachydactyly, in which the middle long bone in the fingers fails to grow and therefore the fingers remain very short. This pedigree, published in 1905, was the first demonstration of dominant Mendelian inheritance in humans. This particular form of brachydactyly results from a mutation in a gene whose normal product is a protein involved in cartilage formation, which is necessary for bone growth.
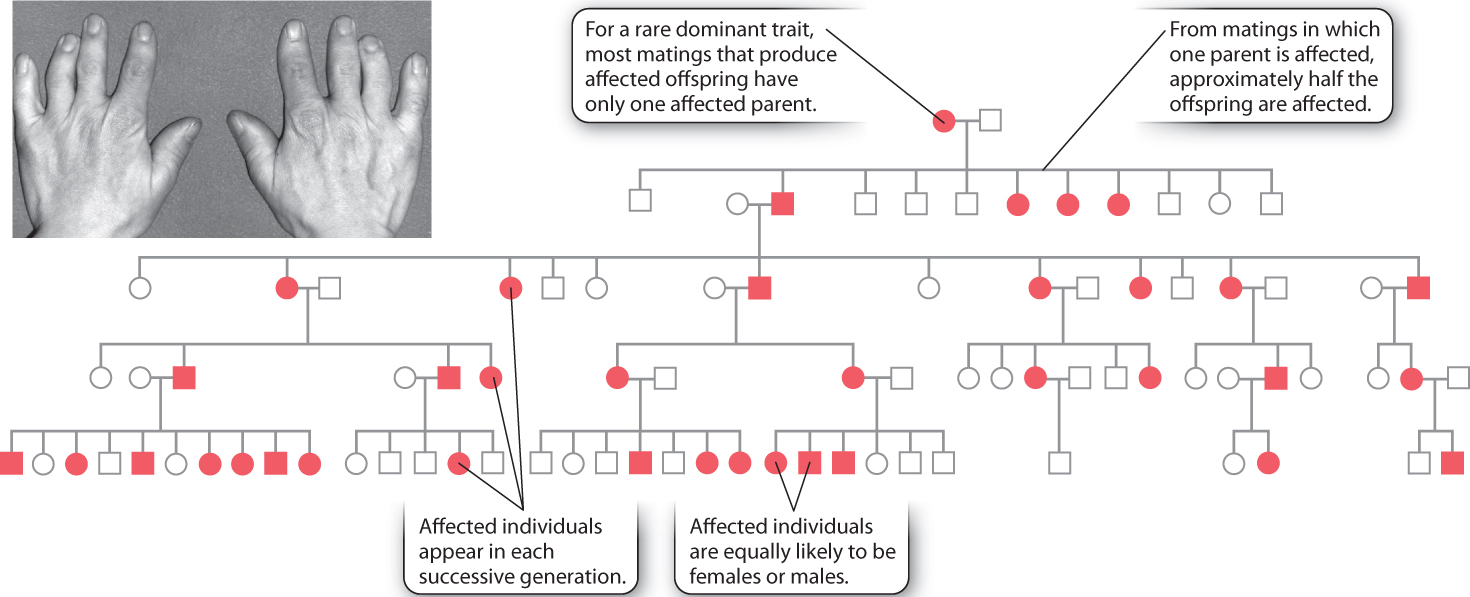
These are the features of the pedigree that immediately suggest dominant inheritance:
- Affected individuals are equally likely to be females or males.
- Most matings that produce affected offspring have only one affected parent. This occurs because the brachydactyly trait is rare, and therefore a mating between two affected individuals is extremely unlikely.
- Among matings in which one parent is affected, approximately half the offspring are affected.
If a dominant trait is rare, then affected individuals will almost always be heterozygous (Aa) and not homozyogous (AA). In a mating in which one parent is heterozygous for the dominant gene (Aa) and the other is homozygous recessive (aa), half the offspring are expected to be heterozygous (Aa) and the other half homozygous recessive (aa).
16-16
16.5.2 Recessive traits skip generations
Recessive inheritance shows a pedigree pattern very different from that of dominant inheritance. The pedigree shown in Fig. 16.19 pertains to albinism, in which the amount of melanin pigment in the skin, hair, and eyes is reduced. In most populations, the frequency of albinism is about 1 in 36,000, but it has a much higher frequency—about 1 in 200—among the Hopi and several other Native American Indian tribes of the Southwest. (It is not unusual for genetic diseases to have elevated frequencies among certain isolated populations.) This type of albinism is due to a mutation in the gene OCA2, which encodes a membrane transporter protein. As noted in Chapter 15, another type of mutation affecting expression of this same gene is associated with blue eyes. As shown in this pedigree, double lines represent matings between relatives, and in both cases shown here the mating is between first cousins.
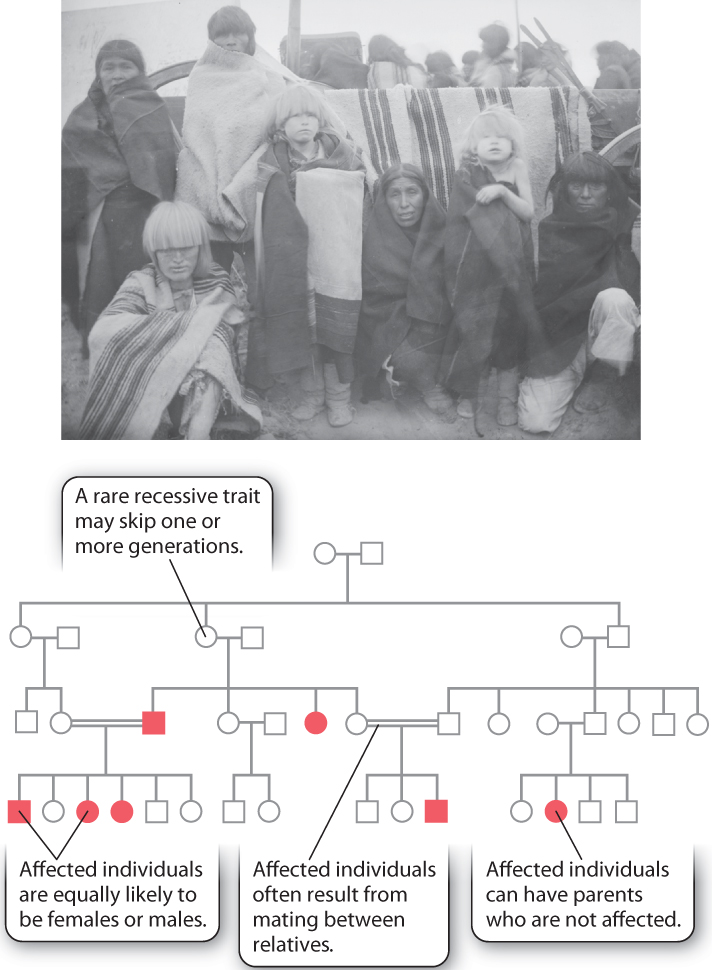
These are the principal pedigree characteristics of recessive traits:
- The trait may skip one or more generations.
- Affected individuals are equally likely to be females or males.
- Affected individuals may have unaffected parents, as in the offspring of the second mating in the second generation in Fig. 16.19. For a recessive trait that is sufficiently rare, virtually all affected individuals have unaffected parents.
- Affected individuals often result from mating between relatives, typically first cousins
Recessive inheritance has these characteristics because recessive genes can be transmitted from generation to generation without manifesting the recessive phenotype. In order for an affected individual to occur, the recessive gene must be inherited from both parents. Mating between relatives often allows rare recessive alleles to become homozygous because an ancestor that is shared between the relatives may carry the gene (Aa). The recessive gene in the common ancestor can be transmitted to both parents, making them each a carrier of the gene as well. If both parents are unaffected carriers of the allele, they both have the genotype Aa, and therefore ¼ of their offspring are expected to be homozygous aa and affected.
16.5.3 Many genes have multiple alleles
The examples discussed so far in this chapter involve genes with only two alleles, such as A for yellow seeds and a for green seeds, or the allele of human OCA2 for normal skin pigmentation and the mutant OCA2 allele for albinism. Because a gene consists of a sequence of nucleotides, however, any nucleotide or set of nucleotides in the gene can undergo mutation. Each of the mutant forms that exists in a population constitutes a different allele, and hence a population of organisms may contain many different alleles of the same gene, which are called multiple alleles. As noted in Chapter 15, some genes have so many alleles that they can be used for individual identification by means of DNA typing.
In considering genetic diseases, multiple alleles are often grouped into categories such as “mutant” and “normal.” But there are often many different “mutant” and many different “normal” alleles in a population. For the “mutant” alleles, the DNA sequences are different from one another, but none of them produces a functional protein product. For the “normal” alleles, the DNA sequences are also different, but they all are able to produce functional protein.
For example, more than 400 different recessive alleles that cause phenylketonuria (PKU) have been discovered across the world. PKU is a moderate to severe form of mental retardation caused by mutations in the gene encoding the enzyme phenylalanine hydroxylase. Children affected with PKU are unable to break down the excess phenylalanine present in a normal diet, and the buildup impairs the development of neurons in the brain. About 1 in 10,000 newborns inherits two mutant alleles, which could be two copies of the same mutant allele or two different mutant alleles, and is affected.
16-17
The “normal” form of the gene encoding phenylalanine hydroxylase also exists in the form of multiple alleles, each of which differs from the others, but nevertheless encodes a functional form of the enzyme.
Question Quick Check 6
KvesnrKVDL/89V/u22p6cIAS7Sq5wkXiF+3j/wMiFzTG2Zxximaa4HYG2WphqavHn0yE5UK69aS+AKHa5MReD7k9aFy0v1SXiqo0jgu6zXK0Wyvul5c/TBFk9vUYV6JfU5JI3AvunPL0bPh7PE8di01Jitc/IGNNDtX1voKar6IFWl6A16.5.4 Incomplete penetrance and variable expression can obscure inheritance patterns
Many traits with single-gene inheritance demonstrate complications that can obscure the expected patterns in pedigrees. Chief among these are traits with incomplete penetrance, which means that individuals with a genotype corresponding to a trait do not actually show the phenotype, either because of environmental effects or because of interactions with other genes. Penetrance is the proportion of individuals with a particular genotype that show the expected trait. If the penetrance is less than 100%, then the trait shows reduced, or incomplete, penetrance. Familial cancers often show incomplete penetrance. For example, retinoblastoma caused by certain mutations in the Rb gene and breast cancer caused by certain mutations in the BRCA1 and BRCA2 genes are incompletely penetrant. That is, some people who inherit a mutation in these genes that predisposes to cancer do not in fact develop cancer. Similarly, some mutations in the apoliprotein E (APOE) gene increase the risk of developing Alzheimer’s disease. However, not everyone who inherits these forms of the gene develops Alzheimer’s disease, so these alleles are also incompletely penetrant.
Another common complication in human pedigrees is variable expressivity, which means that a particular phenotype is expressed with a different degree of severity in different individuals. Don’t confuse variable expressivity with incomplete penetrance. With variable expressivity, the trait is always expressed, though the severity varies; with incomplete penetrance, the trait is either expressed or it is not. Variation among individuals in the expression of a trait can result from the action of other genes, from effects of the environment, or both. An example is provided by deficiency of the enzyme alpha-1 antitrypsin (α1AT) discussed in Chapter 15, which is associated with loss of lung elasticity and emphysema. Among individuals with emphysema due to α1AT deficiency, the severity of the symptoms varies dramatically from one patient to the next. In this case, tobacco smoking is an environmental factor that increases the severity of the disease.
Incomplete penetrance and variable expressivity both provide examples where a given genotype does not always produce the same phenotype, since the expression of genes is often influenced by other genes, the environment, or a combination of the two.
16.5.5 How do genetic tests identify disease risk factors?
Case 3 You from A to T: Your Personal Genome
Your personal genome, as well as that of every human being, contains a unique combination of alleles of thousands of different genes. Most of these have no detectable effects on health or longevity, but many are risk factors for genetic diseases. Molecular studies have discovered particular alleles of genes associated with a large number of such conditions, and the presence of these alleles can be tested. More than a thousand genetic tests have already been deployed, and many more are actively being developed. A genetic test is a method of identifying the genotype of an individual. The tests may be carried out on entire populations or restricted to high-risk individuals.
The benefits of genetic testing can be appreciated by an example. Screening of newborns for phenylketonuria identifies babies with high blood levels of phenylalanine. In the absence of treatment, 95% of such newborns will progress to moderate or severe mental retardation, whereas virtually all those placed on a special diet with a controlled amount of phenylalanine will have mental function within the normal range. For recessive conditions like phenylketonuria, tests can be carried out on people with affected relatives to identify the heterozygous genotypes. Testing can also identify genetic risk factors for disease, and carriers can take additional precautions. For example, individuals with α1AT deficiency can prolong and improve the quality of their lives by not smoking tobacco, women with genetic risk factors BRCA1 and BRCA2 for breast cancer can have frequent mammograms, and those with the TCF7L2 risk factor for type 2 diabetes can decrease their risk by lifestyle choices that include weight control and exercise.
While there are many potential benefits to genetic testing, there are also some perils. One major concern is maintaining the privacy of those who choose to be tested. With medical records increasingly going online, who will have access to your test results, and how will this information be used? Could your test results be used to deny you health or life insurance because you have a higher than average risk of some medical condition? Or could an employer who got hold of your genetic test results decide to reassign you to another job, or even eliminate your position because of your genetic predispositions? There are some safeguards designed to protect you from such discrimination. The Genetic Information Nondiscrimination Act (GINA) was signed into law in 2008 and forbids the use of genetic information in decisions concerning employment and health insurance. The protection provided by GINA will, it is hoped, allow for the responsible and productive use of genetic information.
16-18
There is also increasing concern about the reliability and accuracy of genetic tests, especially direct-to-consumer (DTC) genetic tests. DTC tests can be purchased directly without the intervention of medical professionals. Since the consumer sends a biological sample and DTC tests are carried out by the provider, the tests are not regarded as medical devices and so are unregulated. One problem is that some DTC tests are based on flimsy and unconfirmed evidence connecting a gene with a disease. Another is that the link between genotype and risk may be exaggerated for marketing purposes. Yet another is lack of information on quality control in the DTC laboratories. Finally, consumer misinterpretation may regard genotype as destiny, at one extreme descending into depression and despair, and at the other using a low-risk genotype to justify an unhealthy lifestyle.