17.2 INHERITANCE OF GENES IN THE X CHROMOSOME
Genes in the X chromosome are called X-linked genes. These genes have a unique pattern of inheritance first discovered by Thomas Hunt Morgan in 1910. Morgan’s pioneering studies of genetics of the fruit fly Drosophila melanogaster helped bring Mendelian genetics into the modern era. The discovery of X-linked inheritance was not only important in itself, but also provided the first experimental evidence that chromosomes contain genes.
17.2.1 X-linked inheritance was discovered through studies of male fruit flies with white eyes.
Morgan’s discovery of X-linked genes began when he noticed a white-eyed male in a bottle of fruit flies in which all the others had normal, or wild-type, red eyes. (The most common phenotype in a population is often called the wild type.) This was the first mutant he discovered, and finding it was a lucky break. As we saw in Chapter 16, most mutations are recessive, which means that when they occur their effect on the organism (the phenotype) is not observed because of the presence of the nonmutant gene in the homologous chromosome. Recall that the nonmutant form of a recessive mutant gene is dominant, and that the different forms of the gene are alleles.
Morgan’s initial crosses are outlined in Fig. 17.3. In the first generation, he crossed the white-eyed male with a wild-type red-eyed female. All of the progeny fruit flies had red eyes, as you would expect from any recessive mutation. Morgan then carried out brother–sister matings, and he found that the phenotype of white eyes reappeared among the progeny. This result, too, was expected. However, there was a surprise: Morgan observed that the white-eye phenotype was associated with the sex of the fly. In the second generation, all the white-eyed fruit flies were male, and the white-eyed males appeared along with red-eyed males in a ratio of 1:1. No females with white eyes were observed; all the females had red eyes.
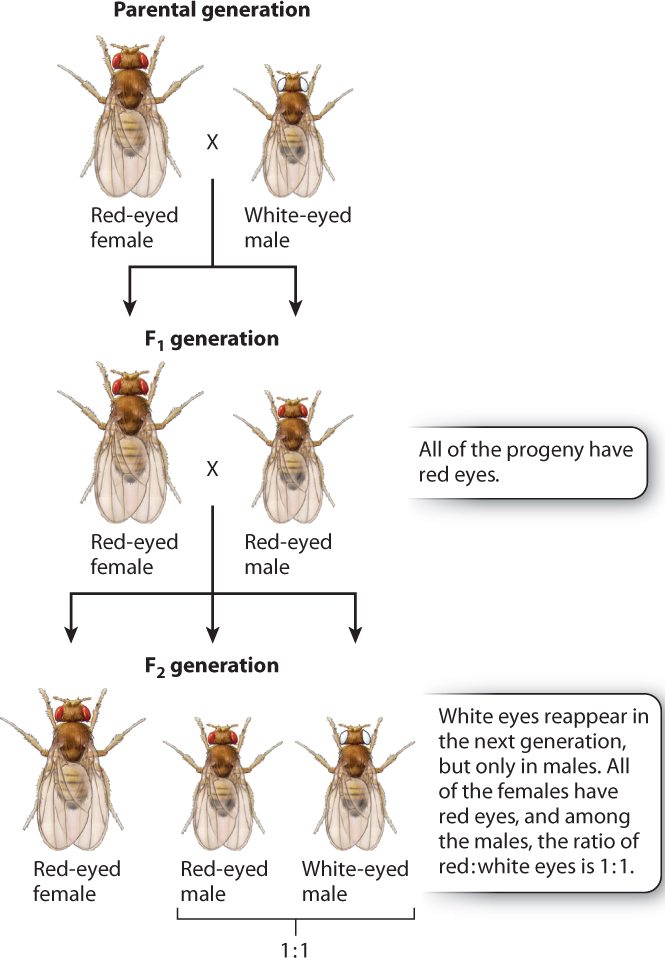
17-4
17.2.2 Genes in the X chromosome exhibit a “crisscross” inheritance pattern.
When Morgan did his crosses with the white-eyed male, the X chromosome had only recently been discovered by microscopic examination of the chromosomes in male and female grasshoppers. Morgan was the first to understand that the pattern of inheritance of the X chromosome would be different from that of the autosomes, and he proposed the hypothesis that the white-eyed phenotype was due to a mutation in a gene in the X chromosome. This hypothesis could explain the pattern of inheritance shown in Fig. 17.3.
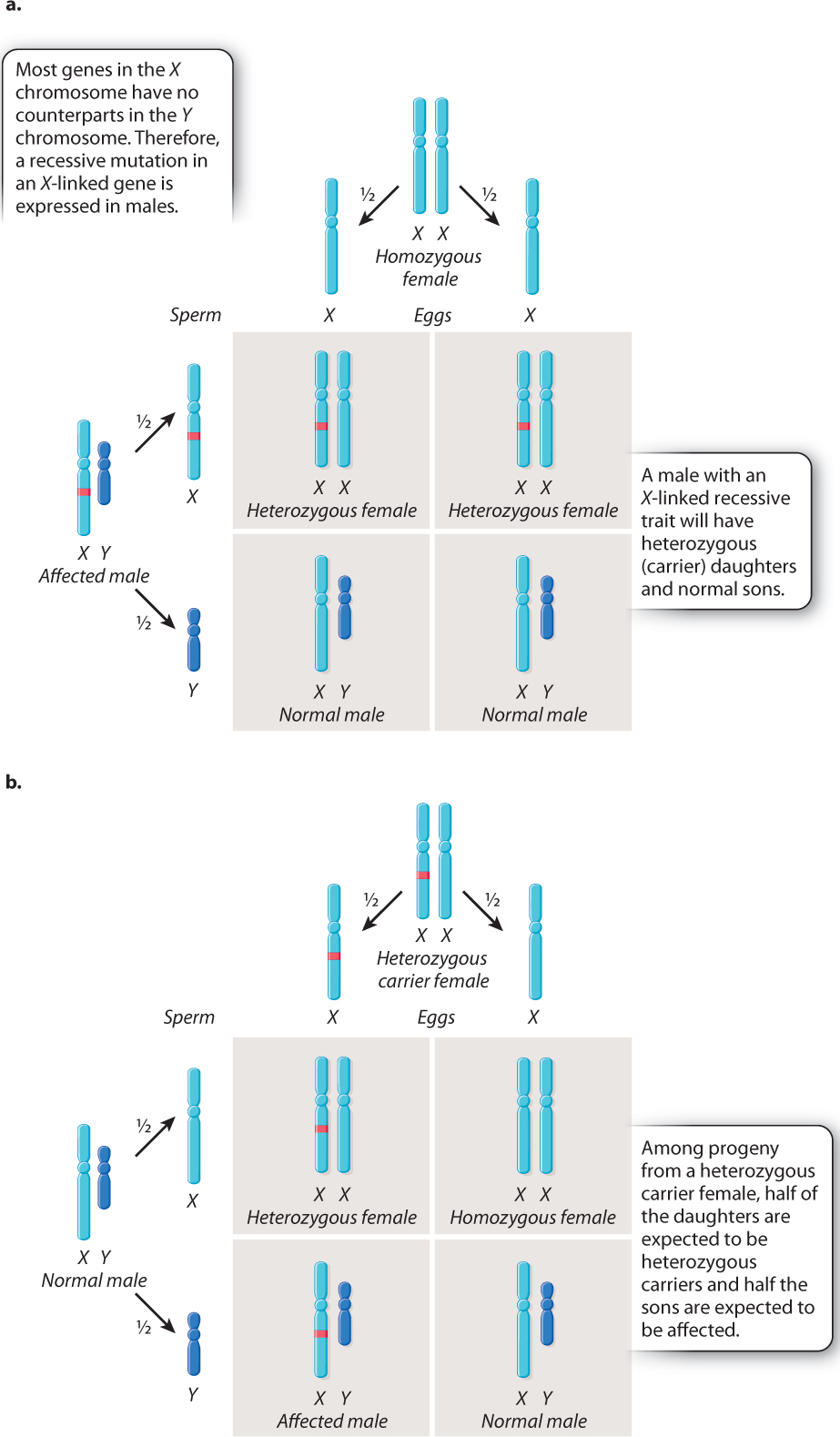
The key features of X-linked inheritance are shown in Fig. 17.4. In Drosophila, as in humans, females are XX and males are XY. Fig. 17.4a shows an XY male in which the X chromosome contains a recessive mutation. Because the Y chromosome does not carry an allele of this gene, the recessive mutation will be reflected in the male’s phenotype—in this case, white eyes.
The Punnett square in Fig. 17.4a explains why all the offspring had red eyes when Morgan crossed the white-eyed male with a red-eyed female. During meiosis in the male, the mutant X chromosome segregates from the Y chromosome, and each type of sperm, X or Y, combines with a normal X-bearing egg. The result is that the female progeny are heterozygous. They have only one copy of the mutant allele, and because the mutant allele is recessive, the heterozygous females do not express the white-eye trait. The male progeny are also red-eyed because they receive their X chromosome from their wild-type red-eyed mother.
The Punnett square in Fig. 17.4a illustrates two important principles governing the inheritance of X-linked genes:
17-5
- The XX offspring indicate that a male transmits his X chromosome only to his daughters. In this case, the X chromosome transmitted by the male carries the white-eye mutation.
- The XY offspring indicate that a male inherits his X chromosome from his mother. In this case, the X chromosome transmitted by the mother carries the nonmutant allele of the gene.
These principles underlie a pattern often referred to as crisscross inheritance: An X chromosome present in a male in one generation must be transmitted to a female in the next generation, and in the generation after that can be transmitted back to a male. Therefore, an X chromosome can “crisscross,” or alternate, between the sexes in successive generations.
The cross illustrated in Fig. 17.4b shows another important feature of X-linked inheritance, one that explains the results of Morgan’s F1 cross. In this case, the mother is a heterozygous female. During meiosis, the mutant allele and the nonmutant allele undergo segregation. Half of the resulting eggs contain an X chromosome with the mutant allele, and half contain an X chromosome with the nonmutant allele. These combine at random with either X-bearing sperm or Y-bearing sperm. Among the female progeny in the next generation, half are heterozygous for the mutant allele and the other half are homozygous for the normal allele. Thus, none of the females exhibits the white-eye trait because the mutation is recessive. Among the male progeny, half receive the mutant allele and have white eyes, whereas the other half receive the nonmutant allele and have red eyes. The expected progeny from the cross in Fig. 17.4b therefore consist of all red-eyed females, and there is a 1:1 ratio of red-eyed to white-eyed males, which is what Morgan observed in the F2 generation of his crosses, as shown in Fig. 17.3.
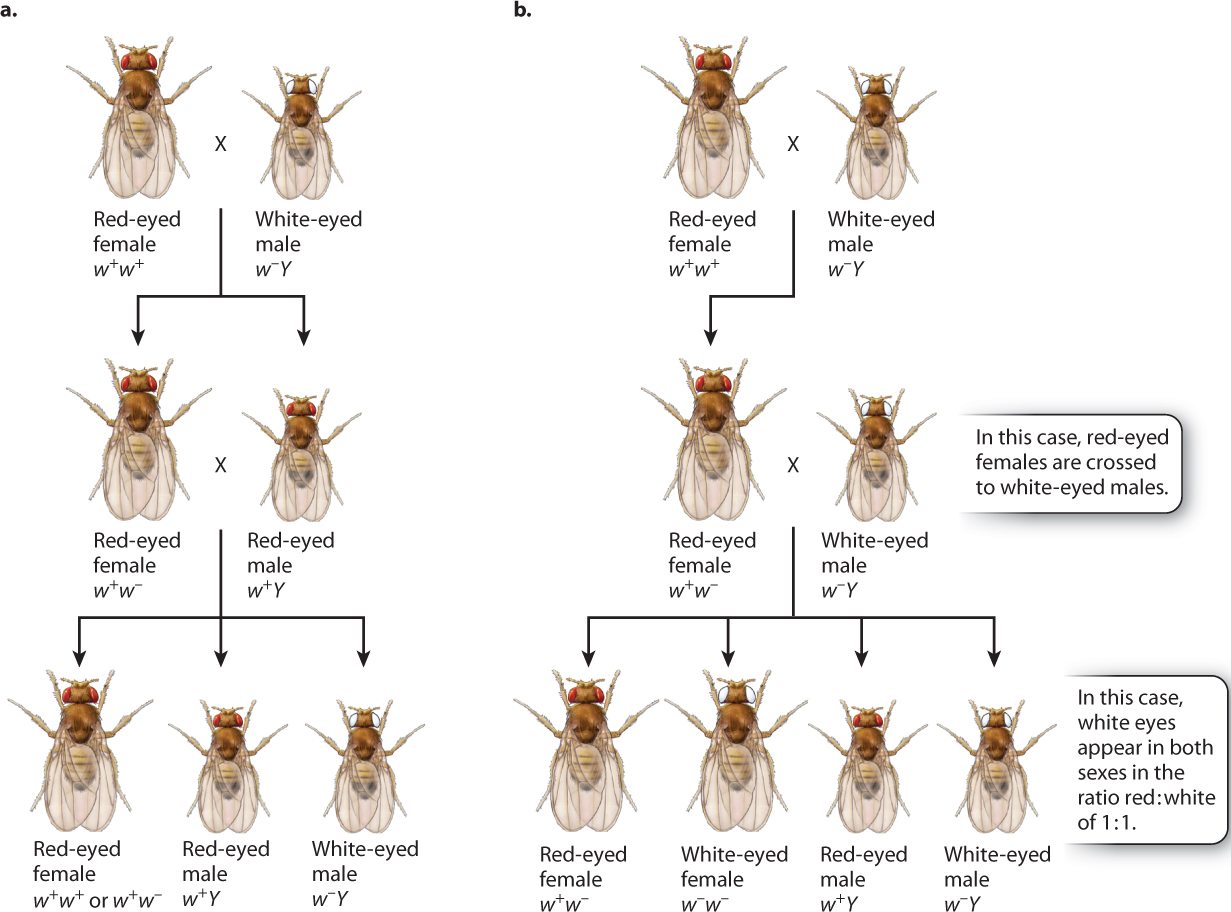
Knowing the patterns revealed by the Punnett squares, we can now assign genotypes to Morgan’s original crosses (Fig. 17.5). In Fig. 17.5, the white-eyed male that Morgan used in his first crosses are given the genotype of w−Y, and the red-eyed female has a genotype of w+w+. The symbol “w−” stands for the recessive white-eye mutation in one X chromosome, and the symbol “w+” stands for the dominant nonmutant allele (red eyes) in the other X chromosome. The symbol “Y” stands for the Y chromosome, and it is important to remember that the Y chromosome does not contain an allele of the white-eye gene.
In the cross between a wild-type red-eyed female and a white-eyed male, illustrated in Fig. 17.5a, the male offspring have red eyes because they receive their X chromosome from their mother. The female offspring from this cross receive one of their X chromosomes from their father, and hence they are heterozygous, w1w2. When the male and female progeny are mated together, their offspring consist of all red-eyed females (half of which are heterozygous) and a 1:1 ratio of red-eyed to white-eyed males, exactly as Morgan had observed.
17-6
The hypothesis of X-linkage not only explained the original data, but it also predicted the results of other crosses. One important test is outlined in Fig. 17.5b. Here the original cross is the same as that in Fig. 17.5a, but instead of mating the hybrid females to their brothers, they are mated to white-eyed males. The prediction is that there should be a 1:1 ratio of red-eyed to white-eyed females as well as a 1:1 ratio of red-eyed to white-eyed males. Again, these were the results observed. By the results of these crosses and others, Morgan demonstrated that the pattern of inheritance of the white-eye mutation parallels the pattern of inheritance of the X chromosome.
17.2.3 X-linkage provided the first experimental evidence that genes are in chromosomes.
Morgan’s original experiments indicated that the white-eye mutation showed a pattern of inheritance like that expected of the X chromosome. However, it was one of Morgan’s students who showed experimentally that the white-eye mutation was actually a physical part of the X chromosome. Today it seems obvious that genes are in chromosomes because we know that genes consist of DNA and that DNA in the nucleus is found in chromosomes. But in 1916, when Calvin B. Bridges, who had joined Morgan’s laboratory as a freshman, was working on his PhD research under Morgan’s direction, neither the chemical nature of the gene nor the chemical composition of chromosomes was known.
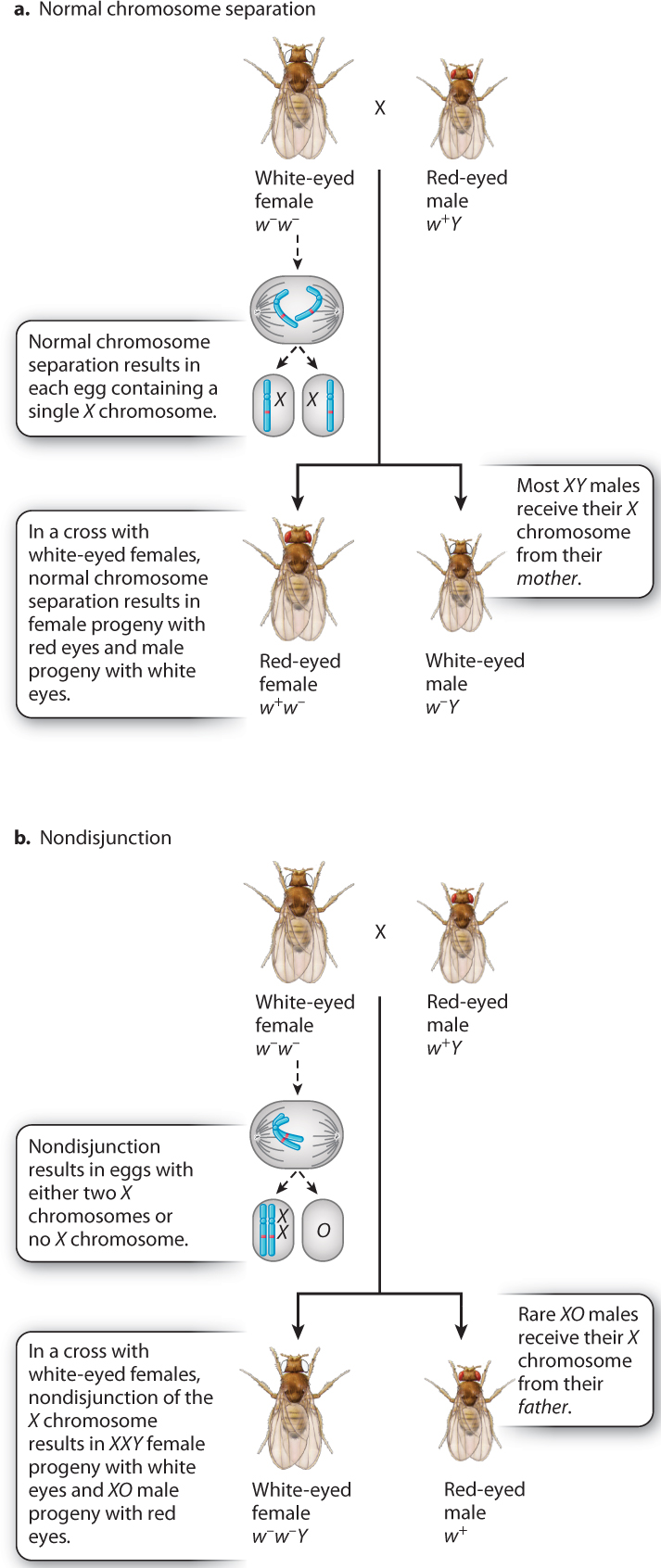
In one set of experiments, Bridges crossed white-eyed females with red-eyed males (Fig. 17.6). Usually, the progeny consisted of red-eyed females and white-eyed males (Fig. 17.6a). This is the result expected when the X chromosomes in the mother separate normally at anaphase I in meiosis because all the daughters receive a w+-bearing X chromosome from their father and all the sons receive a w−-bearing X chromosome from their mother.
But Bridges noted a few rare exceptions. He saw that about 1 offspring in 2000 from the cross was “exceptional”—either a female with white eyes or a male with red eyes. The exceptional females were fertile, and the exceptional males were sterile. To explain these exceptional progeny, Bridges proposed the hypothesis diagrammed in Fig. 17.6b: The X chromosomes in a female occasionally fail to separate in anaphase I in meiosis, and both X chromosomes go to the same spindle pole. Recall from Chapter 15 that chromosomes sometimes fail to separate normally in meiosis, a process known as nondisjunction. Nondisjunction of X chromosomes results in eggs containing either two X chromosomes or no X chromosome. Figure 17.6b shows the implications for eye color in the progeny if the hypothesis is correct. The exceptional white-eyed females would contain two X chromosomes plus a Y chromosome (genotype w−/w−/Y), and the exceptional red-eyed males would contain a single X chromosome and no Y chromosome (genotype w+).
17-7
Bridges’s hypothesis for the exceptional progeny in Fig. 17.6b was bold, as it assumed that Drosophila males could develop in the absence of a Y chromosome (XO embryos yielding sterile males, where “O” indicates absence of a chromosome), and that females could develop in the presence of a Y chromosome (XXY embryos yielding fertile females). The hypothesis was accurate as well as bold. Microscopic examination of the chromosomes in the exceptional fruit flies confirmed that the exceptional white-eyed females had XXY sex chromosomes and that the exceptional sterile red-eyed males had an X but no Y. Because fruit flies with three X chromosomes (XXX) or no X chromosome (OY) were never observed, Bridges concluded that embryos with these chromosomal constitutions are unable to survive. He also showed that nondisjunction can take place in males as well as in females. From the phenotypes of these exceptional fruit flies and their chromosome constitutions, Bridges concluded that the white-eye gene (and by implication any other X-linked gene) is physically present within the X chromosome.
Bridges’s demonstration that genes are present in chromosomes was also the first experimental evidence of nondisjunction. Drosophila differ from humans in that the Y chromosome is necessary for male fertility but not for male development. As we will see later in this chapter, a gene in the Y chromosome itself is the trigger for male development in humans and other mammals, and so for these organisms, the Y chromosome is needed both for male development and male fertility. Nondisjunction occasionally takes place in meiosis in humans as well as in fruit flies. When nondisjunction takes place in the human sex chromosomes, it results in chromosomal constitutions such as 47, XXY and 47, XYY males as well as 47, XXX and 45, XO females. Nondisjunction of autosomes can also occur, resulting in fetuses that have extra copies or missing copies of entire chromosomes. The consequences of nondisjunction of human chromosomes were examined in Chapter 15.
17.2.4 Genes in the X chromosome show characteristic patterns in human pedigrees.
The features of X-linked inheritance can be seen in human pedigrees for traits due to an X-linked recessive mutation. These are illustrated in Fig. 17.7 for red–green color blindness, a condition that affects about 1 in 20 males. An individual with red–green color blindness will have difficulty seeing the number in the colored dots in Fig. 17.7. The key features of X-linked inheritance, noted in the pedigree, are listed here:
- Affected individuals are almost always males because males need only one copy of the mutant gene to be affected, whereas females need two copies to be affected.
- Affected males have unaffected sons because males transmit their X chromosome only to their daughters.
- A female whose father is affected can have affected sons because such a female must be a heterozygous carrier of the recessive mutant allele.
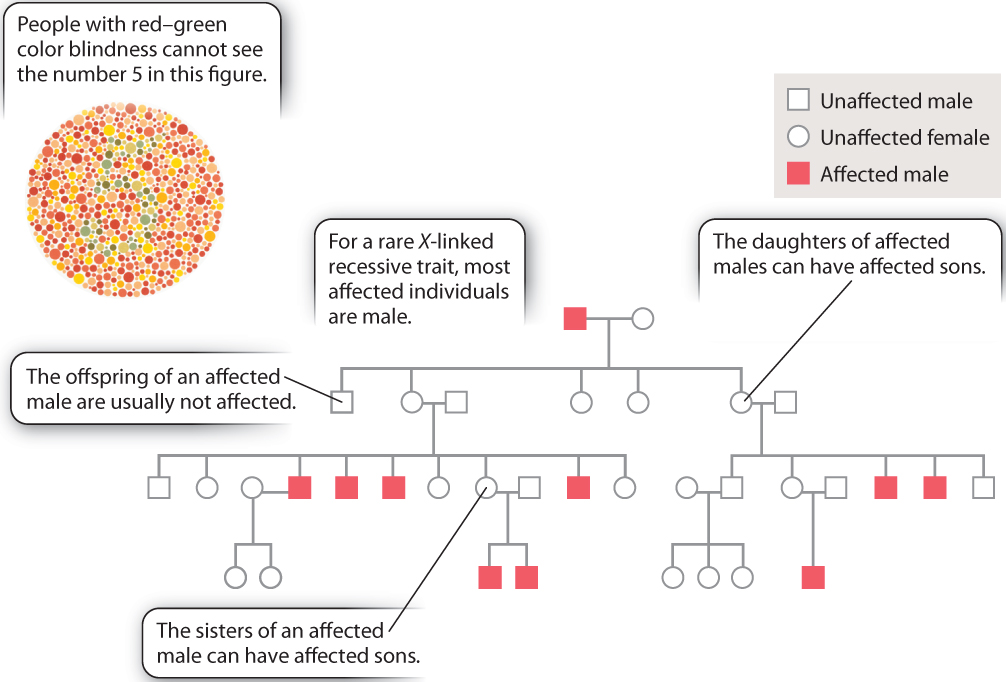
An additional feature worth mentioning is that the sisters of an affected male each have a 50% chance of being a heterozygous carrier because when a brother is affected the mother must be heterozygous for the recessive allele.
Question Quick Check 1
lsWSU5/uqmiYU+hN8DXu3fChKoKEMQCgOGG19FQc291v7VXyXowySJ7dg2928LBPfhXmPh6VxJ24jkgY4SB9WjHH60A7Z5VSvh0aNRzzi0SL1K0XVmYo04OJa6A/6N4+peJIdsuPuI4=17-8
A pedigree for one of the most famous examples of human X-linked inheritance is shown in Fig. 17.8. The trait is hemophilia, which results from a recessive mutation in a gene encoding a protein necessary for blood clotting. Affected individuals bleed excessively from even minor cuts and bruises, and internal bleeding can cause excruciating pain. Affecting about 1 in 7000 males, hemophilia is famous because of its presence in many members of European royalty descended from Queen Victoria of England (1819–1901), who was a heterozygous carrier of the gene. By the marriages of her carrier granddaughters, the gene was introduced into the royal houses of Germany, Russia, and Spain. The mutant allele is not present in the present royal family of England, however, because this family descends from King Edward VII, one of Victoria’s four sons, who was not himself affected and therefore passed only a normal X chromosome to his descendants.
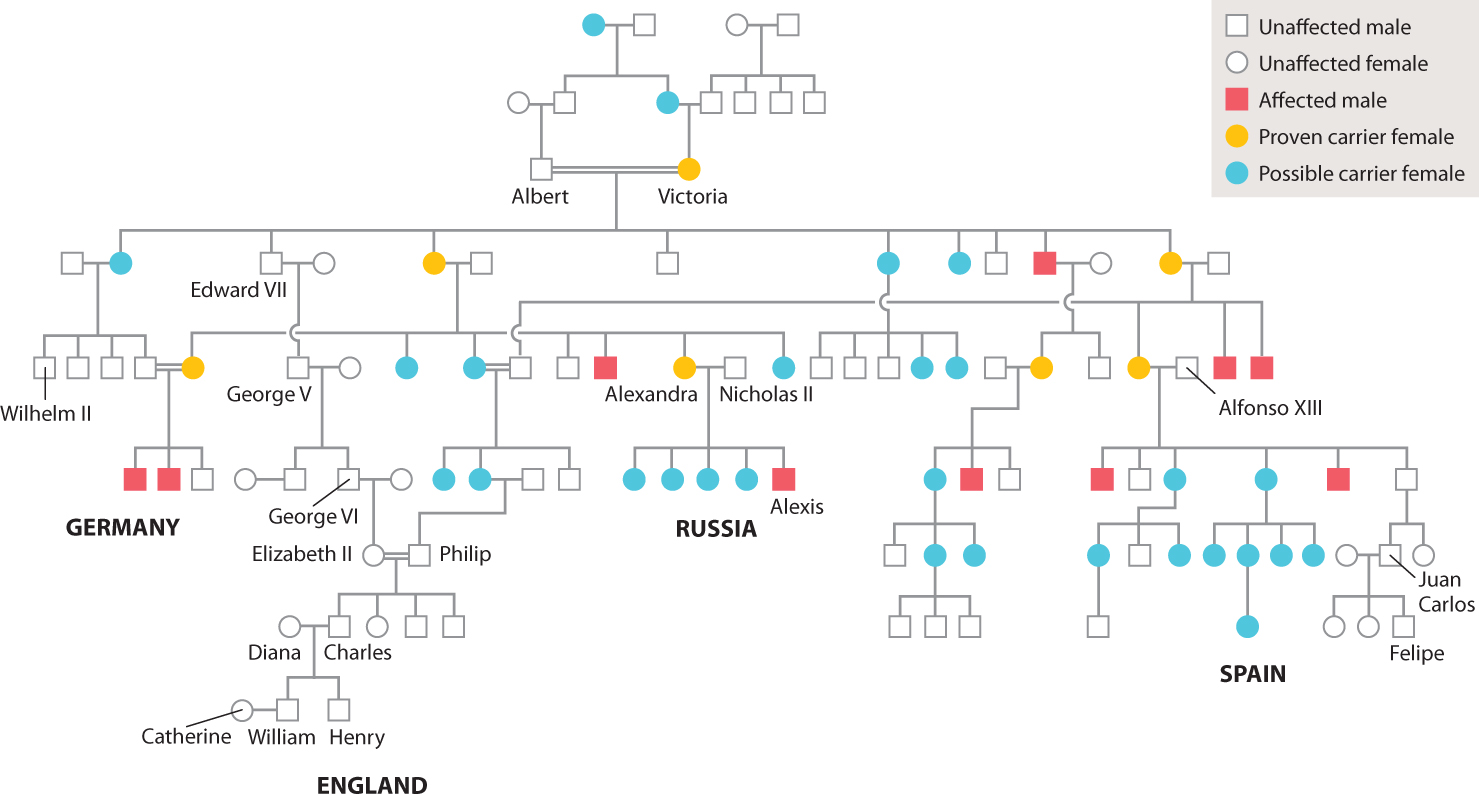
The source of Queen Victoria’s hemophilia mutation is not known. None of her ancestors is reported to have had a bleeding disorder. Quite possibly the mutation was present for a few generations before Victoria was born but remained hidden because it was passed from heterozygous female to heterozygous female.