22.3 SPECIATION
Recognizing that species are groups of individuals that are reproductively isolated from other such groups, we are now in a position to recast the key question: How does speciation occur? Instead of asking, “How do new species arise?” we can ask, “How does reproductive isolation arise between populations?”
22.3.1 Speciation is a by-product of the genetic divergence of separated populations.
The key to speciation is the fundamental evolutionary process of genetic divergence between genetically separated populations. As we saw in Chapter 21, if a single population is split into two populations that are unable to interbreed, different mutations will appear by chance in the two populations. Like all mutations, these will be subject to genetic drift or natural selection, resulting over time in the genetic divergence of the two populations. Two separate populations that are initially identical will, over long periods of time, gradually become distinct as different mutations are introduced and propagated in each population. At some stage in the course of divergence, changes occur in one population that lead to its members being reproductively isolated from members of the other population (Fig. 22.6). It is this process that results in speciation.
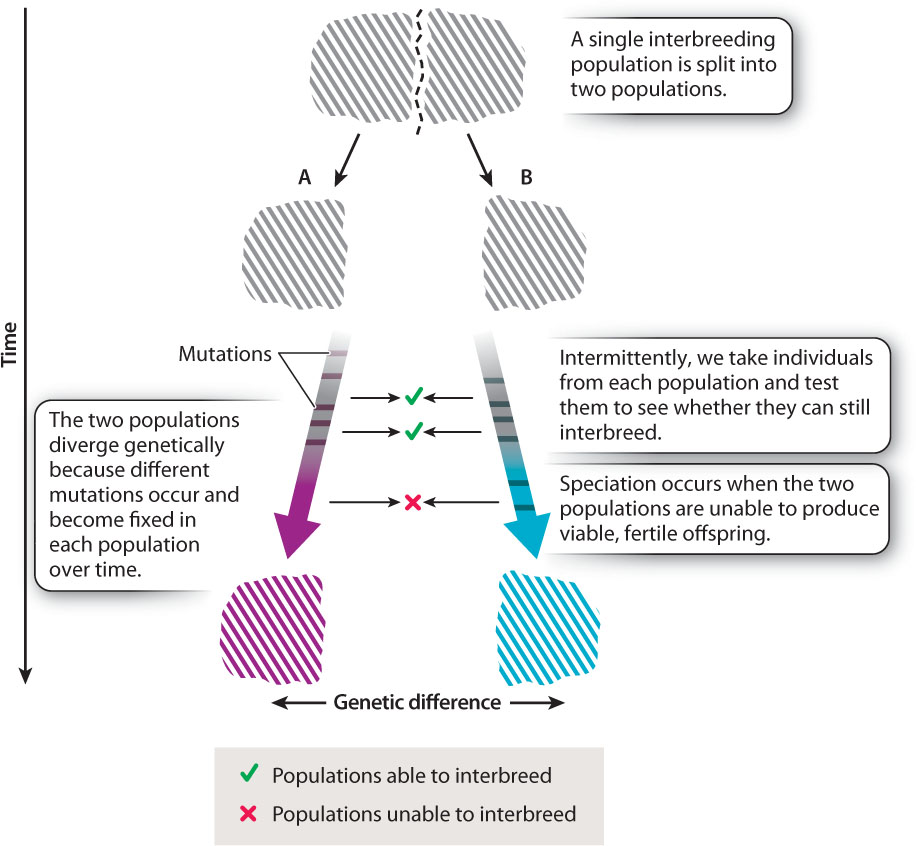
Speciation—the development of reproductive isolation between populations—is, therefore, just a by-product of the genetic divergence of separated populations.
As Fig. 22.6 shows, speciation is typically a gradual process. If we try to cross members of two populations that have genetically diverged but not diverged far enough for full reproductive isolation (that is, speciation) to have arisen, we may find that the populations are partially reproductively isolated. They are not yet separate species, but the genetic differences between them are extensive enough that the hybrid offspring they produce have reduced fertility or viability compared to offspring produced by crosses between individuals within each population.
22.3.2 Allopatric speciation is speciation that results from the geographical separation of populations.
Reproductive isolation between two populations ultimately results in two species. Because this process requires genetic isolation between the diverging populations and because geography is the easiest way to ensure genetic isolation, models of speciation focus on geography.
22-7
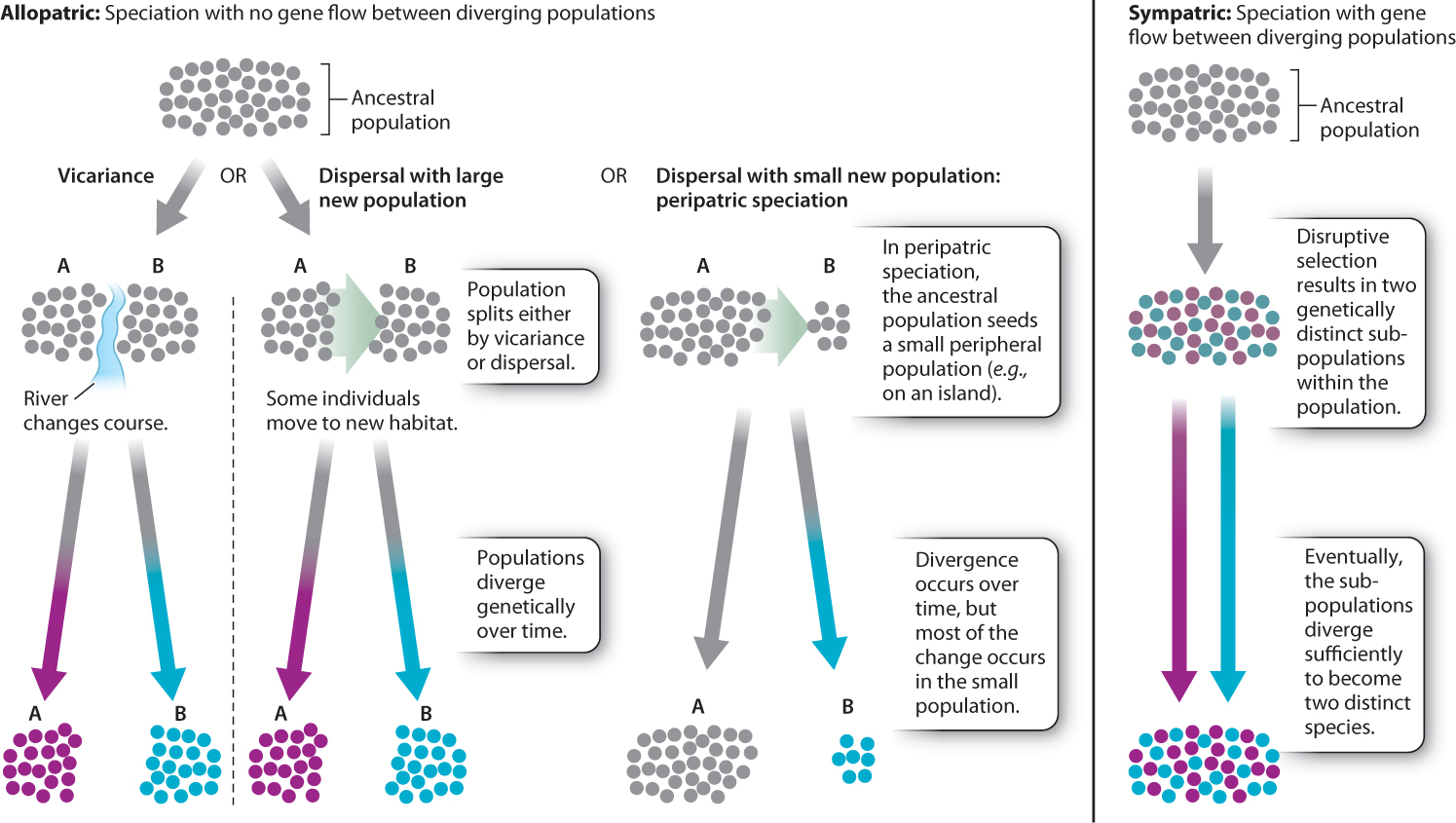
The process usually begins with the creation of allopatric (literally, “different place”) populations, populations that are geographically separated from each other. Clearly, physically separating two populations is the simplest way to ensure that they become genetically isolated from each other. It is therefore not surprising that most speciation is thought to be allopatric. Note, however, that the size and ecology of a species must be considered in deciding whether or not two populations are truly allopatric. Two populations of salamanders separated by a large highway may be allopatric, as salamanders may not be able to cross the highway, whereas such a barrier would obviously not suffice to separate populations of birds. To assess, then, whether two populations are truly allopatric, it is necessary to take more than just location into account.
Because genetic divergence is typically gradual, we often find allopatric populations that have yet to evolve even partial reproductive isolation but which have accumulated a few population-specific traits. This genetic distinctness is sometimes recognized by taxonomists, who deem each geographic form a subspecies by adding a further designation after its species name. For example, Sri Lankan Asian elephants, subspecies Elephas maximus maximus, are generally larger and darker than Indian ones, subspecies Elephas maximus indicus (see Fig. 22.2).
How do populations become allopatric? There are two basic ways. The first is by dispersal, in which some individuals colonize a distant place, such as an island, far from the main source population. The second is by vicariance, in which a geographic barrier arises within a single population, separating it into two or more isolated populations. For example, when sea levels rose at the end of the most recent ice age, new islands formed along the coastline as the low-lying land around them was flooded. The populations on those new islands suddenly found themselves isolated from other populations of their species. This kind of island formation is a vicariance event.
Regardless of how the allopatric populations came about—whether through dispersal or vicariance—the outcome is the same. The two separated populations will diverge genetically until speciation occurs.
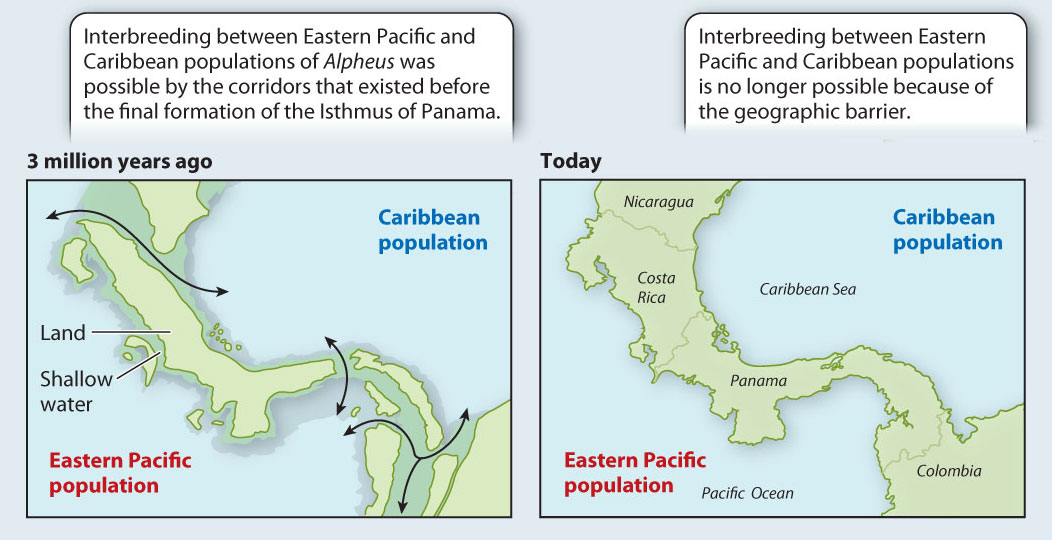
Often, vicariance-derived speciation events are the easiest to study because we can date the time at which the populations were separated if we know when the vicariance event occurred. One such event whose history is well known is the formation of the Isthmus of Panama between Central and South America, shown in Fig. 22.7. This event took place about 3.5 million years ago. As a result, populations of marine organisms in the western Caribbean and eastern Pacific that had formerly been able to interbreed freely were separated from each other. After a period of time, the result was the formation of many distinct species, each of whose closest relatives are on the other side of the isthmus.
FIG. 22.7: Can a vicariance event cause speciation?
BACKGROUND 3.5 million years ago, the Isthmus of Panama was not completely formed. Several marine corridors remained open, allowing interbreeding between marine populations in the Caribbean and the eastern Pacific. Subsequently, the gaps in the isthmus were plugged, separating the Caribbean and eastern Pacific populations and preventing gene flow between them.
HYPOTHESIS Patterns of speciation will reflect the impact of the vicariance event resulting from the closing of the direct marine connections between the Pacific and the Caribbean. Specifically, researchers predicted that each ancestor species (from the time before the formation of the isthmus) split into two “daughter” species, one in the Caribbean and the other in the Pacific. The closest relative of each current Pacific species, then, is predicted to be a Caribbean species (and vice versa).
EXPERIMENT This study focused on 17 species of snapping shrimp in the genus Alpheus, a group that is distributed on both sides of the isthmus. The first step was to sequence the same segment of DNA from each species. The next step was to compare those sequences in order to reconstruct the phylogenetic relationships among the species.
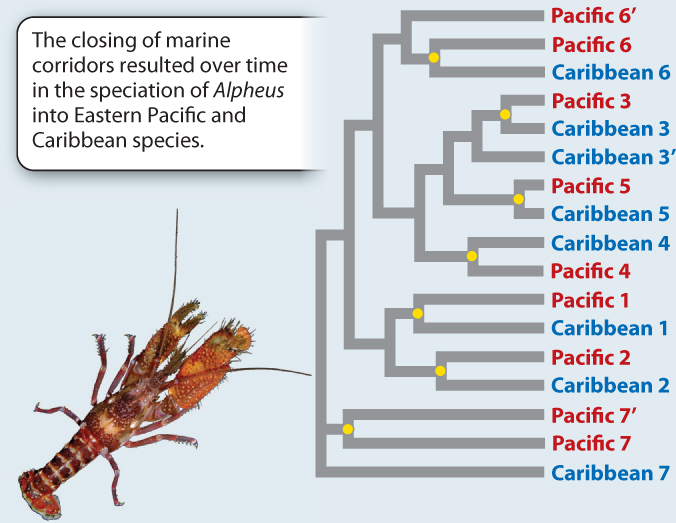
RESULTS The phylogeny reveals that species show a distinctly paired pattern of relatedness: The closest relative of each species is one from the other side of the isthmus.
CONCLUSION That we see these consistent Pacific/Caribbean sister species pairings strongly supports the hypothesis that the vicariant event caused by the formation of the isthmus has driven speciation in Alpheus. Each Pacific/Caribbean pairing is derived from a single ancestral species (indicated by a yellow dot) whose continuous distribution between the Caribbean and eastern Pacific was disrupted by the formation of the isthmus. Here we see striking evidence of the role of vicariance in speciation.
FOLLOW-UP WORK Speciation is about more than just genetic differences between isolated populations, as shown here. Knowlton and colleagues also tested the different species for reproductive isolation and found that there were high levels of isolation between Caribbean/Pacific pairs. Given that we know that each species pair has been separated for at least 3 million years, we can then calibrate the rate at which new species are produced.
SOURCE Knowlton, N., L. A. Weigt, L. A. Solórzano, D. K. Mills, and E. Bermingham. 1993. “Divergence in Proteins, Mitochondrial DNA, and Reproductive Compatibility Across the Isthmus of Panama.” Science 260 (5114):1629–1632.
Dispersal is important in a specific kind of allopatric speciation known as peripatric speciation. In this model, a few individuals from a mainland population (the central population of a species) disperse to a new location remote from the original population and evolve separately. This may be an intentional act of dispersal, or it could be an accident brought about by, for example, an unusual storm that blows migrating birds off their normal route. The result is a distant, isolated island population. “Island” in this case may refer to a true island—like Hawaii—or may simply refer to a patch of habitat on the mainland that is appropriate for the species but is geographically remote from the initial mainland population’s habitat area. For a species adapted to life on mountaintops, a new island might be another previously uninhabited mountaintop. For a rainforest tree species, that new island might be a patch of lowland forest on the far side of a range of mountains that separates it from its mainland forest population.
22-8
22-9
The island population is classically small and often in an environment that is slightly different from that of the mainland population. The peripatric speciation model suggests that change accumulates faster in these peripheral isolates than in the large mainland populations, both because genetic drift is more pronounced in smaller populations than in larger ones and because the environment may differ between the mainland and island in a way that results in natural selection driving differences between the two populations. These mechanisms cause genetic divergence of the island population from the mainland one, ultimately leading to speciation.
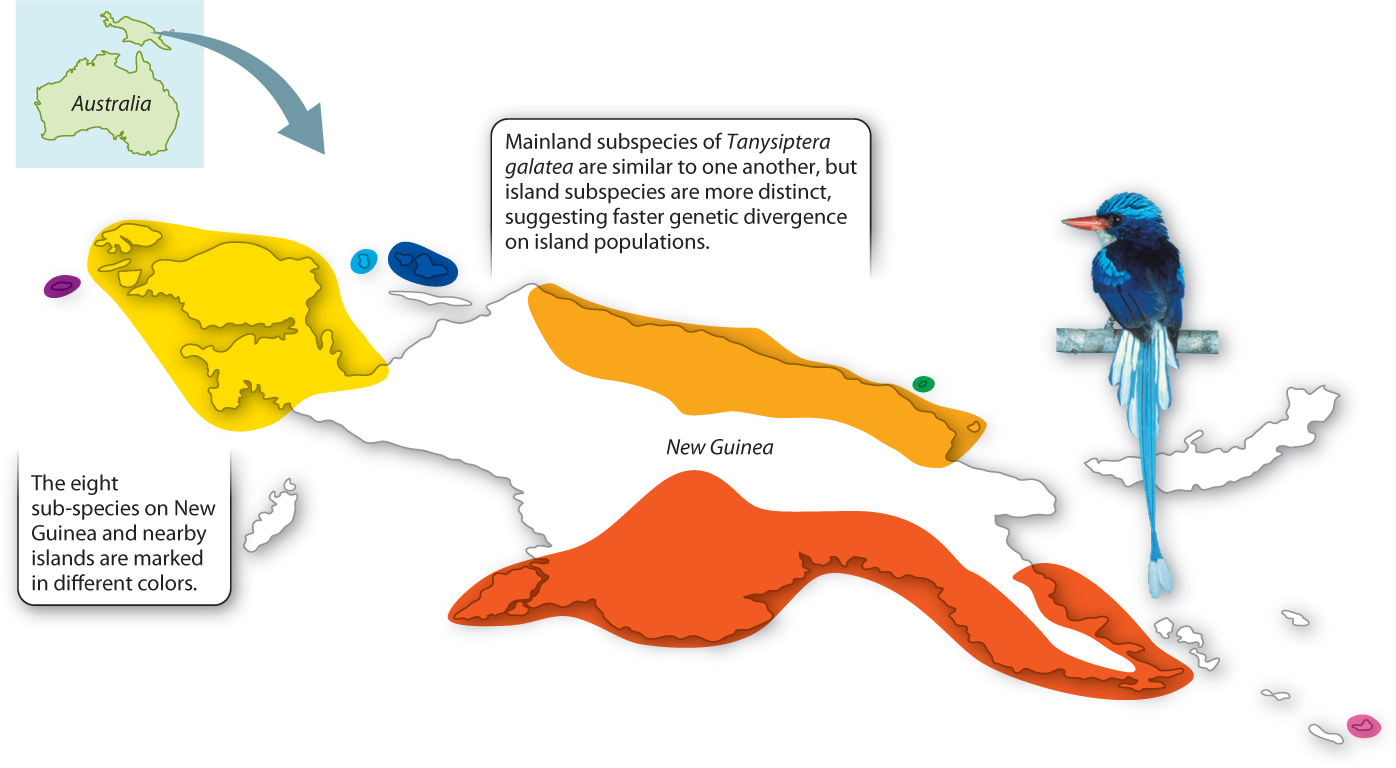
It is possible to glimpse peripatric differentiation in action (Fig. 22.8). Studies of a kingfisher, Tanysiptera galatea, in New Guinea and nearby islands show it under way. There are eight recognized subspecies of T. galatea, three on mainland New Guinea (where they exist in large populations separated by mountain ranges) and five on nearby islands (where, because the islands are small, the populations are correspondingly small). The mainland subspecies are still quite similar to one another, but the island subspecies are much more distinct, suggesting that genetic divergence is occurring faster in the small island populations. If we wait long enough, these subspecies will probably diverge into new species.
Because dating such dispersal events is tricky—newcomers on an island tend not to leave a record of when they arrived—we can some times use vicariance information to study the timing of peripatric speciation. For instance, we know that the oldest of the Galápagos Islands were formed 4–5 million years ago by volcanic action. Some time early in the history of the Galápagos, individuals of a small South American finch species arrived there. Conditions on the Galápagos are very different from those on the South American continent, where the mainland population of this ancestral finch lived, and so the isolated island population evolved to become distinct from its mainland ancestor and eventually became a new species. The finches’ subsequent dispersal among the other islands of the Galápagos has promoted further peripatric speciation (Fig. 22.9). The result was the evolution of 13 different species of finches, known today as Darwin’s finches.
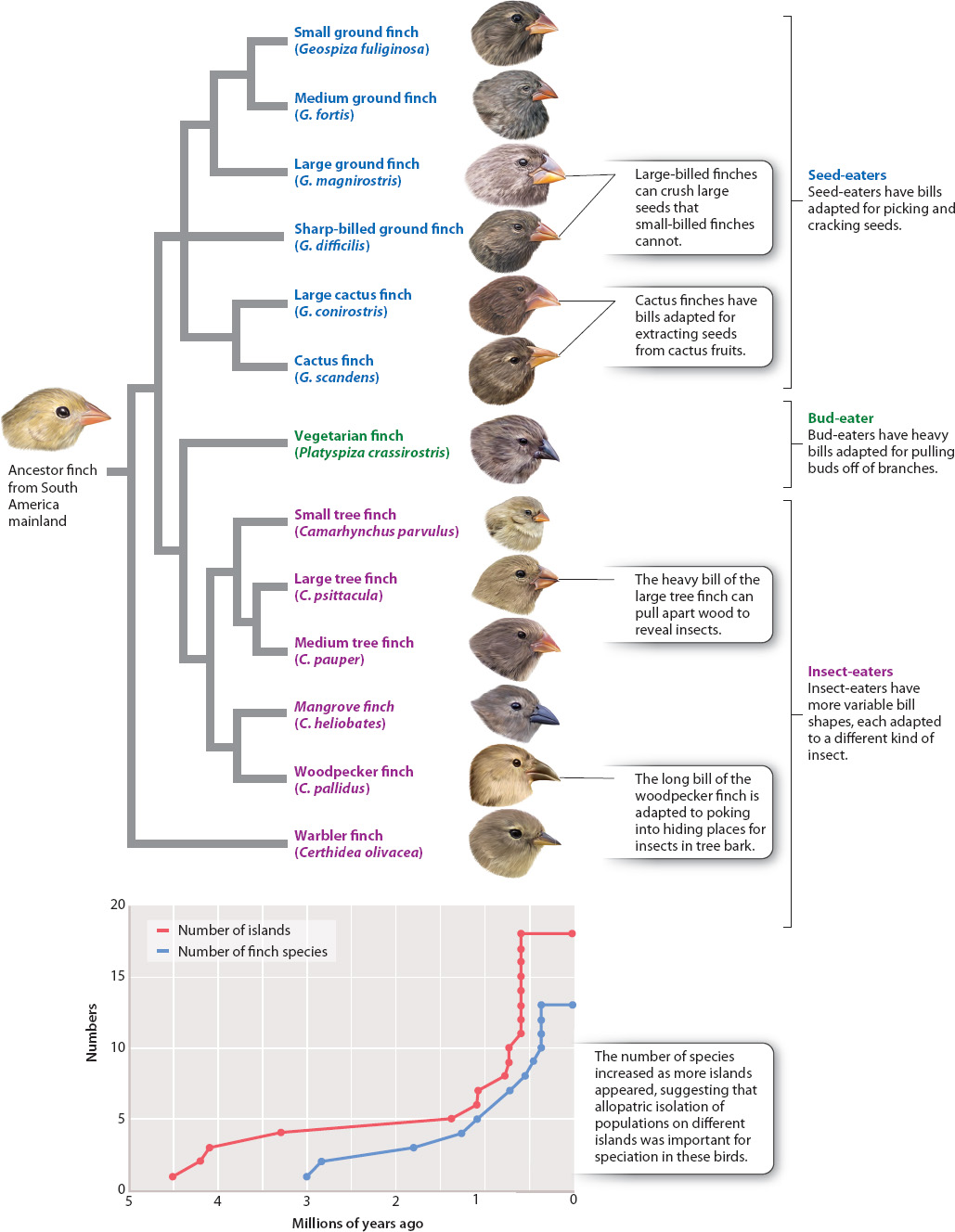
The Galápagos finches and their frenzy of speciation illustrate the important evolutionary idea of adaptive radiation, a bout of unusually rapid evolutionary diversification in which natural selection accelerates the rates of both speciation and adaptation. Adaptive radiation occurs when there are many ecological opportunities available for exploitation. Consider the ancestral finch immigrants arriving on the Galápagos. A wealth of ecological opportunities was open and available. Until the arrival of the colonizing finches, there were no birds on the islands to eat the plant seeds, or to eat the insects on the plants, and so on. Suppose that the ancestral finches fed specifically on medium-sized seeds on the South American mainland (that is, they were medium-seed specialists, with bills that are the right size for handling medium seeds). On the mainland, they were constrained to that size of seed because any attempt to eat larger or smaller ones brought them into conflict with other species—a large-seed specialist and a small-seed specialist—that already used these resources. In effect, stabilizing selection (Chapter 21) was operating on the mainland to eliminate the extremes of the bill-size spectrum in the medium-seed specialists. A somewhat smaller bill, which is good for eating slightly smaller seeds, was selected against because its possessors competed poorly against a small-billed species that is a small-seed specialist. A slightly larger bill was similarly disadvantageous because its possessors ended up competing for large seeds with a species that is a large seed specialist.
22-10
22-11
When the medium-billed immigrant finches first arrived on the Galápagos, however, no such competition existed. Therefore, natural selection promoted the formation of new species of small-and large-seed specialists from the original medium-seed-eating ancestral stock. With the elimination of the competition-mediated stabilizing selection that kept the medium-billed finches medium billed on the mainland, selection actually operated in the opposite direction, favoring the large and small extremes of the bill-size spectrum because these individuals could take advantage of the abundance of unused resources, small and large seeds. It is this combination of emptiness—the availability of ecological opportunity—and the potential for allopatric speciation that results in adaptive radiation.
Question Quick Check 2
Z0QxkY9RZyWWilNytI3HedZyEPl6ei/TeweeCvrPWAPdxEbSF4VVa/YIu//yoqT+dddIRowrk8/TlFfNFArhxI5fzoCNtQhyn7o/FBvU5ewISz2Sd5gXwz6b37MwN3Nk4dAnPin9MV7frvmlgSHhPzOHES8PH+IFD+wuBg==22.3.3 Co-speciation is speciation that occurs in response to speciation in another species.
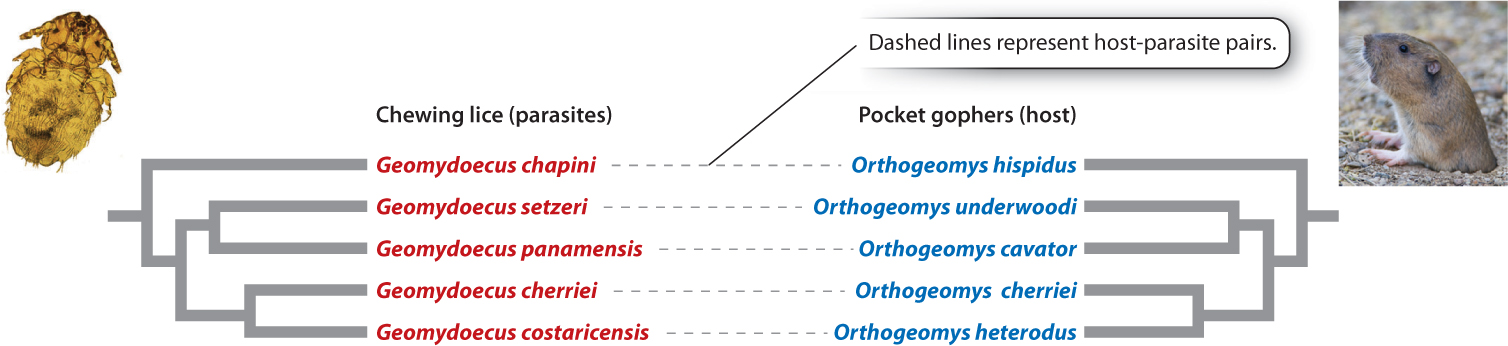
As we have seen, physical separation is often a critical ingredient in speciation. Two populations that are not fully separated from each other—that is, there is gene flow between them—will typically not diverge from each other genetically, because genetic exchange homogenizes them. This is why most speciation is allopatric. Allopatric speciation brings to mind populations separated from each other by stretches of ocean or deserts or mountain ranges. However, separation can be just as complete even in the absence of geographic barriers. Consider an organism that parasitizes a single host species. Suppose that the host undergoes speciation, producing two daughter species. The original parasite population will also be split into two populations, one for each host species. Thus, the two new parasite populations are physically separated from each other and will diverge genetically, ultimately undergoing speciation. This divergence results in a pattern of coordinated host–parasite speciation called co-speciation, a process in which two groups of organisms speciate in response to each other and at the same time.
Phylogenetic analysis of lineages of parasites and their hosts that undergo co-speciation reveals trees that are similar for each group. Each time a branching event—that is, speciation—has occurred in one lineage, a corresponding branching event has occurred in the other (Fig. 22.10).
22.3.4 How did malaria come to infect humans?
Case 4 Malaria: Co-Evolution of Humans and a Parasite
Now let’s look at a human parasite, Plasmodium falciparum, the causative agent of malaria. It had been suggested that P. falciparum’s closest relative is another Plasmodium species, P. reichenowi, found in chimpanzees, our closest living relative. Maybe P. falciparum and P. reichenowi were the products of co-speciation. When the ancestral population split millions of years ago to give rise to human and chimpanzee lineages, that population’s parasitic Plasmodium population could also have been split, ultimately yielding P. falciparum and P. reichenowi.
22-12
Recent studies, however, have disproved this hypothesis. We now know that P. falciparum was introduced to humans relatively recently from gorillas. Why doesn’t the evolutionary history of Plasmodium follow the classical host–parasite co-speciation pattern? We know that this history is complex and is still being unraveled. However, we also know that the mosquito-borne phase of its life cycle facilitates transfer to new hosts. Malaria parasites are thus not as inextricably tied to their hosts as the pocket gopher lice in Fig 22.10. Presumably, it was just such a mosquito-mediated event that resulted in the introduction of P. falciparum into an ancestral human population in Africa from gorillas.
22.3.5 Can sympatric populations—those not geographically separated—undergo speciation?
Can speciation occur without complete physical separation of populations? Evolutionary biologists are still exploring this question. Recall how separated populations inevitably diverge genetically over time (see Fig. 22.6). If a mutation arises in population A after it has separated from population B, that mutation is present only in population A and may eventually become fixed (100% frequency) in that population, either through natural selection, if it is advantageous, or through drift, if it is neutral. Once the mutation is fixed in population A, it represents a genetic difference between populations A and B. Repeated independent fixations of different mutations in the two populations result over time in the genetic divergence of separated populations.
Now imagine that populations A and B are not completely separated and there is some gene flow between them. The mutation that arose in population A can, in principle, appear in population B. The new mutation may indeed have become fixed in population A, but a migrant from population A to population B may introduce the mutation to population B as well. Gene flow effectively negates the genetic divergence of populations. If there is gene flow, a pair of populations may change over time, but they do so together. How, then, can speciation occur if gene flow exists? The term we use to describe populations that are in the same geographic location is sympatric (literally, “same place”). So we can rephrase the question in technical terms: Can speciation occur sympatrically?
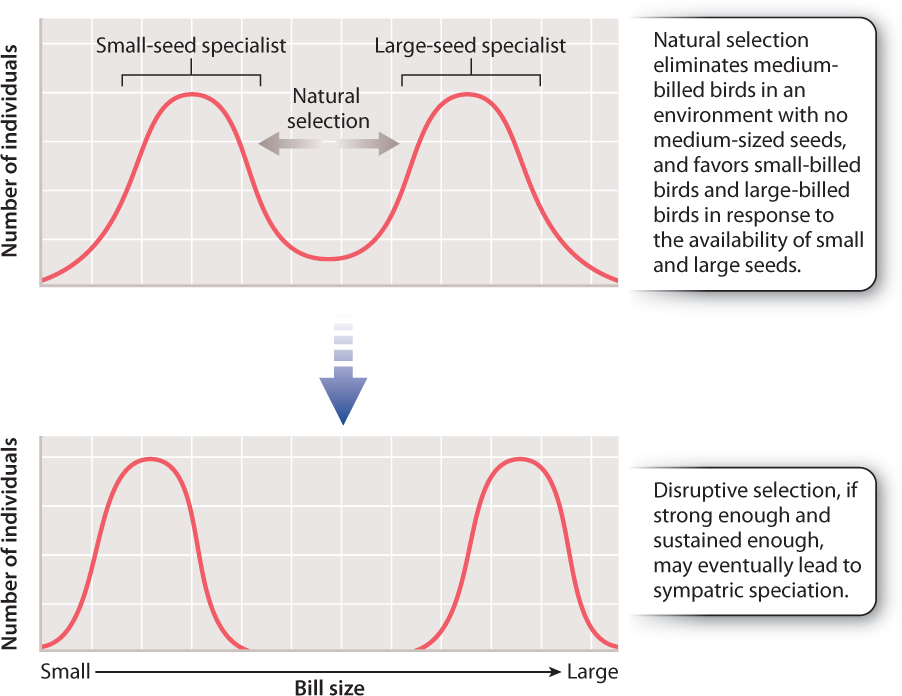
One school of thought insists that sympatric speciation cannot occur and that all speciation is allopatric. If speciation can and does occur sympatrically, one point is clear: Natural selection must act strongly to counteract the homogenizing effect of gene flow. Consider two sympatric populations of finch-like birds, represented in the graph in Fig. 22.11. One population begins to specialize on small seeds and the other on large seeds. If the two populations freely interbreed, no genetic differences between the two will occur and speciation will not take place. Now suppose that the offspring produced by the pairing of a big-seed specialist with a small-seed specialist is an individual best adapted to eat medium seeds, and there are no medium-sized seeds available in the environment. Natural selection will act against the hybrids, which will starve to death because there are no medium-sized seeds for them to eat and they are not well adapted to compete with the big-or small-seed specialists. Natural selection would then, in effect, eliminate the products of gene flow. So, although gene flow is occurring, it does not affect the divergence of the two populations because the hybrid individuals do not survive to reproduce. As discussed in Chapter 21, this form of natural selection, which operates against the middle of a spectrum of variation, is called disruptive selection.
Is there evidence for sympatric speciation? It turns out to be difficult to find evidence of sympatric speciation in nature, though it may not be especially rare in plants, as we will see. We might find two very closely related species in the same location and argue that they must have arisen through sympatric speciation. However, there is an alternative explanation. One species could have arisen elsewhere by, for example, peripatric speciation and subsequently moved into the environment of the other one. In other words, the speciation occurred in the past in allopatry, and the two species are only currently sympatric because of migration after speciation occurred.
22-13
However, recent studies of fish in isolated lakes have provided strong, if not definite, evidence of sympatric speciation. In the volcanic mountains of Cameroon in West Africa, there are several small crater lakes inhabited by a diverse array of fish. In each case, all the fish species in a single lake can be shown to be one another’s closest relatives. Presumably, all the species in a lake are descended from a single common ancestor, the fish species that first arrived in the lake. Each lake is small, so fish populations within them share a single habitat, implying that the speciation events that must have occurred to generate the current species diversity were sympatric.
This demonstration that sympatric speciation has in all probability occurred shows, at the least, that sympatric speciation is possible. We must recognize that we do not yet know just how much of all speciation is sympatric and how much is allopatric. Fig. 22.12 summarizes modes of speciation based on geography.
Question Quick Check 3
wiZP5E/EK/qspZmGAZg1UgdO4FTdJB+eTbVplrFiS1ioUtYMXhmBYlMf/szPlZxsyKkatN1RHyglmUu4gVzkaBCGzsRqEkg8cCEpdeIG0fPLaSJvofPHpPJ2yim2utde47pZpXPXnNzjapiISU/5ixiOU4HAJkElbw4rbsWgVTUtY359BGNkEhbLkEZ3+31yiv2BvXQn25gj6ax2vSjRD68wDHvTfU3zG28V7AXLNu0fkl0rMD6KPTjFGW2WAe2Jh9r4QxyEkV481tzpmAPlSbfdxa1WfrkEcpsf1T3S+uchvRiivwjQhTf9W85y3xUqlJKlKiCdFof88qa7XyBn+FGPpkTzdIUgnKi/yI4I9NO2bQLF+h8P00MtwundYmEqGOc5sIIouc9fUCbQeuMKNVzzHedkbb/86zBN9R1630rZe03aGvSZt5wvGP8kYosalQtbQ3qZlfJ9Gksx22.3.6 Speciation can occur instantaneously.
Although speciation is typically a lengthy process, it can occasionally occur in a single generation, making it sympatric by definition. Typically cases of such instantaneous speciation are caused by hybridization between two species in which the offspring are reproductively isolated from both parents.
For example, hybridization in the past between two sunflower species, Helianthus annuus and H. petiolaris (the ancestor of the cultivated sunflower) has apparently given rise to three new sunflower species, H. anomalus, H. paradoxus, and H. deserticola (Fig. 22.13). H. petiolaris and H. annuus have probably formed innumerable hybrids in nature, virtually all of them inviable. However, a few—the ones with a workable genetic complement—are the ones that survived to yield these three daughter species. In this case, each one of these new species has acquired a different mix of parental chromosomes. It is this species-specific chromosome complement that makes all three distinct and reproductively isolated from the parent species and from one another.
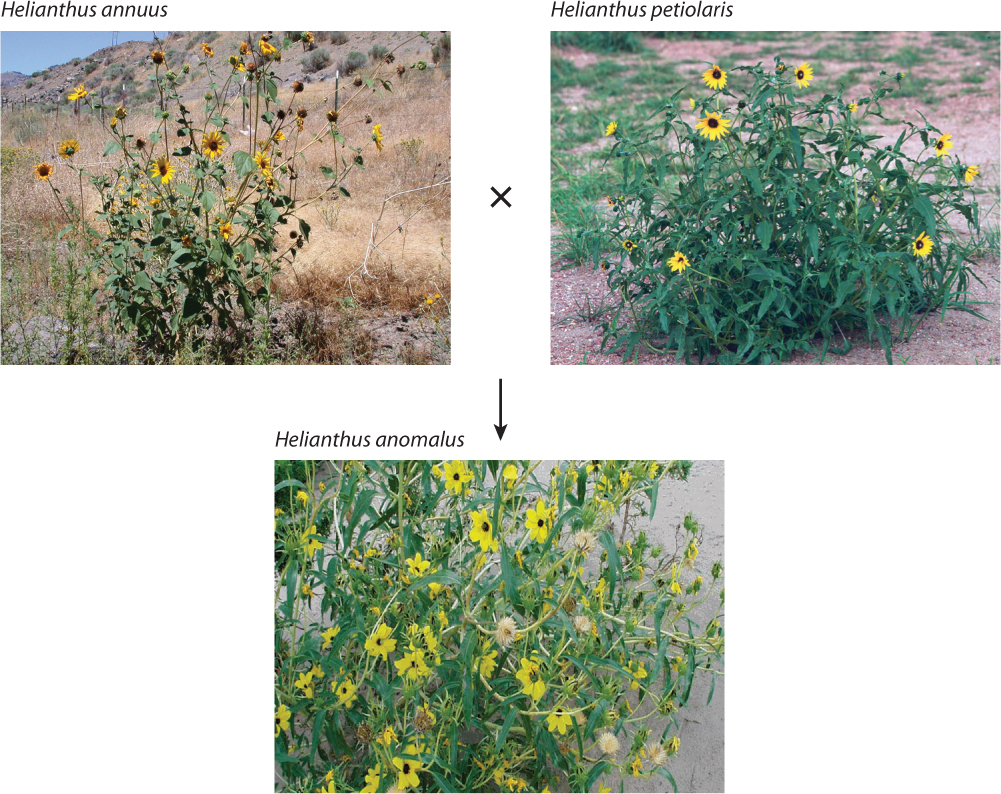
22-14
In many cases of hybridization, chromosome numbers may change. Two diploid parent species with 5 pairs of chromosomes, for a total of 10 chromosomes each, may produce a hybrid with double the number of chromosomes (that is, the hybrid inherits a full paired set of chromosomes from each parental species)—a total of 20. In this case, the hybrid has four genomes rather than the diploid number of two. We call such a double diploid a tetraploid. In general, animals cannot sustain this kind of expansion in chromosome complement, but plants are more likely to do so. As a result, the formation of new species through polyploidy—multiple chromosome sets (Chapter 13)—has been relatively common in plants.
Polyploids may be allopolyploids, meaning that they are produced from hybridization of two different species. For example, related species of Chrysanthemum appear, on the basis of their chromosome numbers, to be allopolyploids. Alternatively, polyploids may be autopolyploids, meaning that they are derived from an unusual reproductive event between members of a single species. In this case, through an error of meiosis in one or both parents, the chromosome number is again increased. However, in contrast to the situation with allopolyploids, all the chromosomes derive from the single parental species. For example, Anemone rivularis, a plant in the buttercup family, has 16 chromosomes, and its close relative A. quinquefolia has 32. A. quinquefolia appears to be an autopolyploid derived from the joining of A. rivularis gametes with two full sets of chromosomes each.
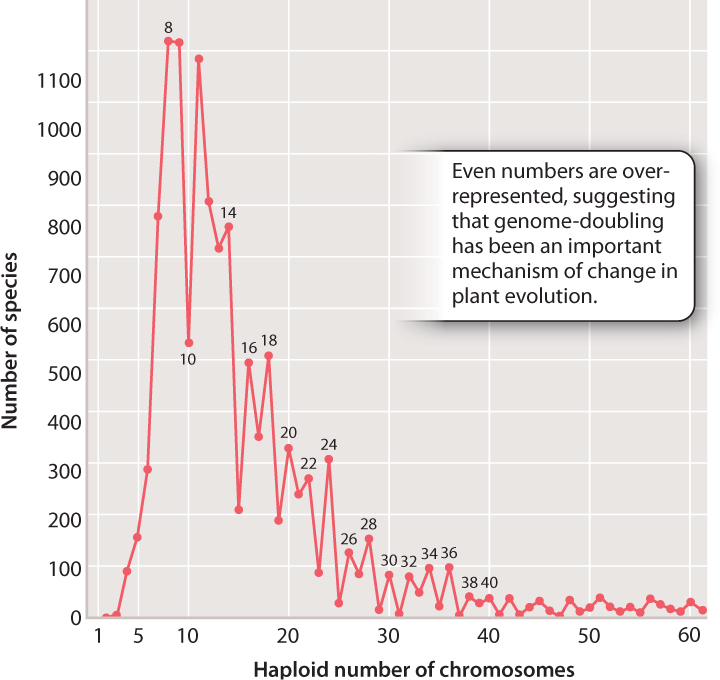
So rampant is speciation by polyploidy in plants that it affects the pattern of chromosome numbers across all plants. In Fig. 22.14, we see the haploid chromosome numbers of thousands of plant species plotted on a graph. Note that, as the numbers get higher, even numbers tend to predominate, suggesting that the doubling of total chromosome complement (a form of polyploidy that will always result in an even number of chromosomes) is an important factor in plant evolution.
22-15