24.4 HUMAN GENETIC VARIATION
So far we have treated humans as all alike, and in many ways, of course, we are. But there are also many differences from one person to the next. Those differences ultimately have two sources: genetic variation and differences in environment (Chapter 18). A person may be born with dark skin, or a person born with pale skin may acquire darker skin—a tan—in response to exposure to sun.
The differences we see from one person to the next are deceptive. Despite appearances, ours is not the most genetically variable species on Earth. While it is certainly true that everyone alive today (except for identical twins) is genetically unique, our species is actually rather low in overall amounts of genetic variation. Modern estimates based on comparisons of many human DNA sequences indicate that, on average, about 1 in every 1000 base pairs differs among individuals (that is, our level of DNA variation is 0.1%). That’s about 10 times less genetic variation than in fruit flies (which nevertheless all look the same to us) and about two to three times less than in Adélie penguins, which look strikingly similar to one another (see Fig. 21.1).
Why, then, are we all so phenotypically different if there is so little genetic variation in our species? Given the large size of our genome, a level of variation of 0.1% translates into a great many genetic differences. Our genome consists of approximately 3 billion base pairs, so 0.1% variation means that 3 million bp differ between any two people chosen at random. Many of those differences are in noncoding DNA, but some fall in regions of DNA that encode proteins and therefore influence the phenotype, so there is a fairly large reservoir of genetic variation in humans. When those mutations are reshuffled by recombination, we get the vast array of genetic combinations present in the human population.
24-13
24.4.1 The prehistory of our species has had an impact on the distribution of genetic variation.
The reasons for our species’ relative lack of genetic variation compared to other species lie in prehistory, and factors affecting the geographical distribution of that variation also lie in the past. Studies of the human family tree initiated by Rebecca Cann’s original mtDNA analysis are giving a detailed picture of how our ancestors colonized the planet and how that process affected the distribution of genetic variation across populations today. Detailed analyses of different populations, often using mtDNA or Y chromosomes, allow us to reconstruct the history of human population movements.
As we have seen, Homo sapiens arose in Africa. Perhaps 60,000 years ago, populations started to venture out through the Horn of Africa and into the Middle East (Fig. 24.15). The first phase of colonization took our ancestors through Asia and into Australia by about 50,000 years ago. Not until about 15,000 years ago did the first modern humans cross from Siberia to North America to populate the New World.
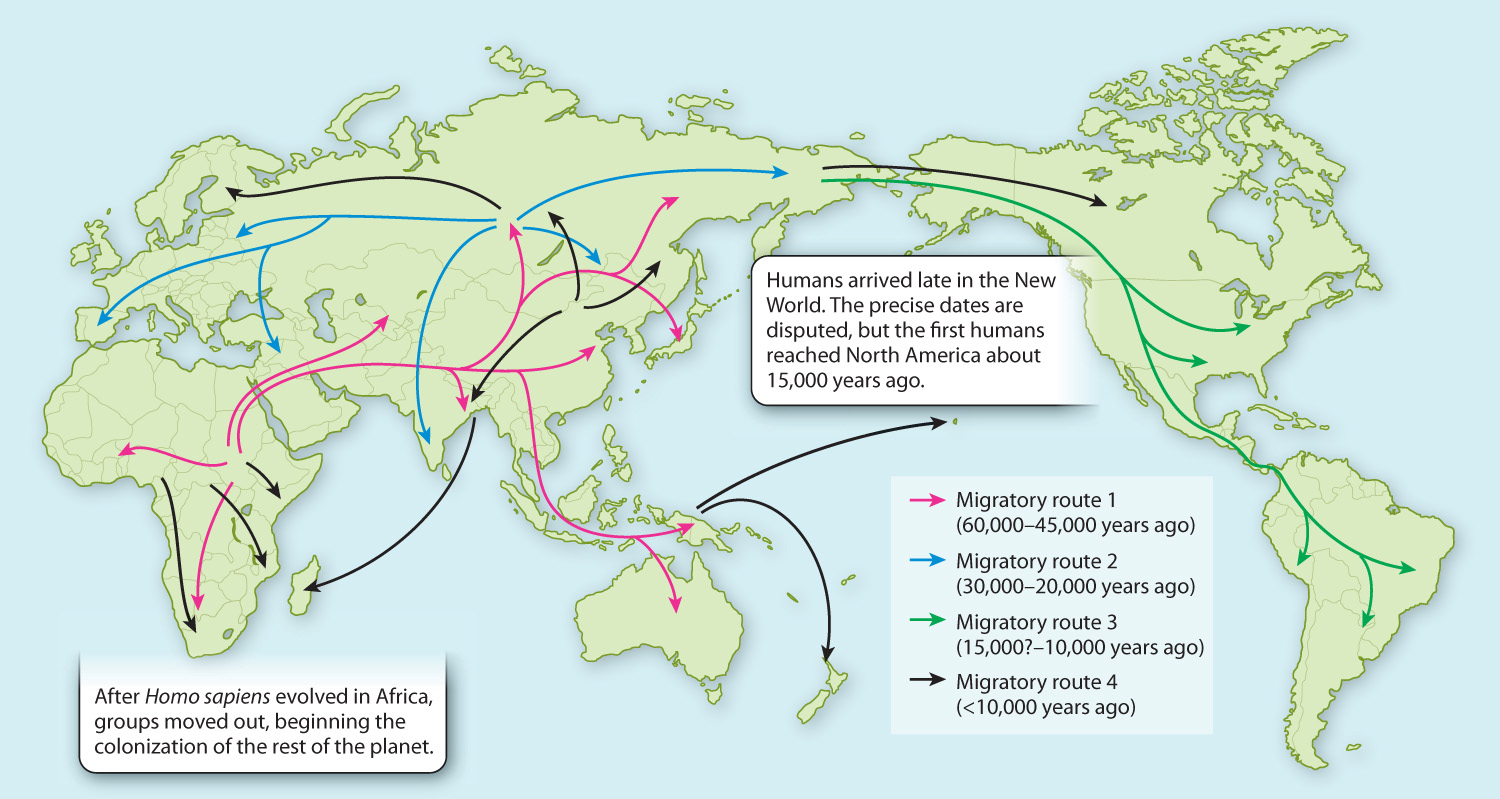
Other colonizations were even later. Despite its closeness to the African mainland, the first humans arrived in Madagascar only about 2000 years ago, and the colonists came from Southeast Asia, not Africa. Madagascar populations to this day bear the genetic imprint of this surprising Asian input. The Pacific Islands were among the last habitable places on Earth to be colonized during the Polynesians’ extraordinary seaborne odyssey from Samoa, which began about 2000 years ago. Hawaii was colonized about 1500 years ago, and New Zealand only 1000 years ago.
By evolutionary standards, the beginning of the spread of modern humans out of Africa about 60,000 years ago is very recent. There has therefore been relatively little time for differences to accumulate among regional populations, and most of the variation in human populations today arose in ancestral populations before any humans left Africa. When we compare levels of variation in a contemporary African population to that in a non-African population, like Europeans or Asians, we find there is more variation in the African population. This is because the individuals that left Africa 60,000 years ago to found populations in the Middle East and beyond were a relatively small sample of the total amount of genetic variation then present in the human population. Non-African populations therefore began with less genetic variation, and that initial lower variation is reflected in their genetic profiles today.
24-14
24.4.2 The recent spread of modern humans means that there are few genetic differences between groups.
Because the out-of-Africa migration was so recent, the genetic differences we see among geographical groups—sometimes called races—are minor. This fact is highly counterintuitive. We see many superficial differences between an African and a Caucasian, such as skin color, facial form, and hair type, and assume that these superficial differences must reflect extensive genetic differences. This assumption made sense when the standard theory about the origin of modern humans was the multiregional one. If European and African populations really had been geographically isolated from each other for as long as 2 million years, then we would expect significant genetic differences between the populations.
We expect isolated populations to diverge genetically over time as different mutations occur in each population. The longer two populations have been isolated from each other, the more genetic differences between them we expect to see (Chapter 21). Isolation lasting 2 million years implies that the differences are extensive, but isolation of 60,000 years suggests they are relatively few. Patterns of genetic variation among different human populations support the hypothesis that human populations dispersed as recently as 60,000 years ago. What we see when we look at genetic markers—variable A’s, T’s, G’s, and C’s in human DNA—is that there is indeed very little genetic differentiation by what sometimes is called race.
In short, there’s a disconnect: Different groups may look very different, but, from a genetic perspective, they’re not very different at all. Statistical analyses have shown that approximately 85% of the total amount of genetic variation in humans occurs within a population (for example, Swedes); 8% occurs between populations within races (for example, in Caucasians, between Swedes and Italians); and the remaining 7% occurs between races. The characteristics we use when we assess an individual’s ethnicity, such as skin color, eye type, and hair form, are encoded by genetic variants that lie in that 7%. If Earth were threatened with destruction and only one population—Swedes, for example—survived, 85% of the total amount of human genetic variation that exists today would still be present in that Swedish population.
24.4.3 Some human differences have likely arisen by natural selection.
We can conclude that the genetic variants affecting traits we can easily see are overrepresented in the 7% of human genetic variation that sorts by race. If we look at other genetic variants, ones that don’t affect traits that we can see, there is no racial pattern. An African is as likely to have a particular base-pair mutation in a gene as a European. So why are visible traits so markedly different among races and others are not? Given the short amount of time (by evolutionary standards) since all Homo sapiens were in Africa, it is likely that the differences we see between groups are the product of selection.
People with dark skin tend to live in lower latitudes with high levels of solar radiation, and people with light skin tend to live in higher latitudes with low levels of solar radiation. It is likely that natural selection is responsible for the physical differences between these populations. Assuming that the ancestors of non-African populations were relatively dark-skinned, what selective factors can account for the loss of pigmentation?
A likely factor is an essential vitamin, vitamin D, which is particularly important in childhood because it is needed for the production of bone. A deficiency of vitamin D can result in the skeletal malformation known as rickets. The body can synthesize vitamin D, but the process requires ultraviolet radiation. Heavily pigmented skin limits the entry of UV radiation into cells and so limits the production of vitamin D. This does not present a difficulty in parts of the world where there is plenty of sunlight, but it can be problematical in regions of low sunlight. Presumably, natural selection favored lighter skin in the ancestors of Eurasian populations because lighter skin favored the production of the vitamin.

Some aspects of body shape and size may also have been influenced by natural selection. In hot climates, where dissipating body heat is a priority, a tall and skinny body form has evolved. Exemplified by East African Masai, this body type maximizes the ratio of surface area to volume and thus aids heat loss. In colder climates, by contrast, selection has favored a more robust, stockier body form, as exemplified by the Inuit, who have a low ratio of surface area to volume that promotes the retention of heat (Fig. 24.16). In these two cases, these are plausible explanations of body form. We should bear in mind, however, that simple one-size-fits-all explanations of human difference are almost always simplistic. Our species is complex and diverse—and often defies generalizations.
Attempts have been made to identify the adaptive value of obvious visual differences between races, such as facial features. It’s possible that natural selection played a role in the evolution of these differences, but an alternative explanation, one originally suggested by Charles Darwin, is more compelling: sexual selection.
As we have seen, there is an apparent mismatch between the extent of difference among groups in visible characters, such as facial features, and the overall level of genetic difference between human groups. Sexual selection can account for this mismatch because it operates solely on characteristics that can readily be seen, such as the peacock’s tail. As we learn more about the genetic underpinnings of the traits in question, we will be able to investigate directly the factors responsible for the differences we see among groups. Sexual selection is discussed in Chapter 45.
24-15
24.4.4 What human genes are under selection for resistance to malaria?
Case 4 Malaria: Co-Evolution of Humans and a Parasite
We see evidence of regional genetic variation in response to local challenges, especially those posed by disease. Malaria, for example, is largely limited to warm climates because it is transmitted by a species of mosquito that can survive only in these regions. Historically, the disease has been devastating in Africa and the Mediterranean. As we saw in Chapter 21, the sickle allele of hemoglobin, S, has evolved to be present at high frequencies in these regions because in heterozygotes it confers some protection against the disease. But in homozygotes, the S allele is highly detrimental because it causes sickle-cell anemia. Homozygotes for the allele encoding normal hemoglobin are also at a disadvantage because they are entirely unprotected from the parasite.
The S allele is beneficial only in the presence of malaria. If there is no malaria in an area, the S allele is disadvantageous, so natural selection presumably acted rapidly to eliminate it in the ancestors of Europeans when they arrived in malaria-free regions. The continued high frequency of the S allele in Africans, some Mediterranean populations, and in populations descended recently from Africans (such as African-Americans) is, however, a reflection of the response of natural selection to a regional disease.
The hemoglobin genes are not the only genes that are under selection for resistance to malaria. Glucose-6-phosphate dehydrogenase (G6PD), a gene involved in glucose metabolism, is one of several other genes implicated. People who are heterozygotes for a mutation in the G6PD gene—and therefore have a G6PD enzyme deficiency—can develop severe anemia when they eat certain foods (most notably fava beans; hence, the condition is called favism). People who are heterozygotes for a mutation in the G6PD gene, however, also have increased resistance to malaria, apparently because they are better at clearing infected red blood cells from their bloodstream. In areas where malaria is common, the advantage of malaria resistance offsets the disadvantage of favism.
Detailed evolutionary analysis of mutations in G6PD shows that favism has arisen multiple times, each time selectively favored because of its role in the body’s response to the malaria parasite. As expected, favism, like sickle-cell anemia, is mainly a feature of populations in malarial areas or of populations whose evolutionary roots lie in these areas.