26.2 AN EXPANDED CARBON CYCLE
In our discussion of energy metabolism in Chapters 7 and 8 and of the carbon cycle in Chapter 25, we emphasized oxidation–reduction (redox) chemistry. In redox reactions, a pair of molecules reacts, one molecule becoming more oxidized and the other becoming more reduced. If we follow the transfer of electrons in a redox reaction, we see that the molecule that is reduced gains electrons, whereas the molecule that is oxidized loses electrons. Reduction therefore requires a source of electrons, or an electron donor, and oxidation requires a sink for electrons, or an electron acceptor.
In photosynthesis, as we discussed in Chapter 8, carbon dioxide (CO2) is reduced to carbohydrates, and water is oxidized to oxygen gas (O2). Water is the electron donor needed to reduce CO2, generating O2 as a by-product. In respiration, as described in Chapter 7, organic molecules are oxidized to CO2, and O2 is reduced to water. In this case, O2 serves as the electron acceptor needed to oxidize organic molecules.
In all known photosynthetic eukaryotes, the photosynthetic reaction is oxygenic, or oxygen producing. There is a good reason for this. Water occurs nearly everywhere and, in the oxygen-rich environments where most eukaryotic organisms thrive, no other molecule is available to donate electrons. Similarly, essentially all respiration in eukaryotic cells is aerobic, or oxygen utilizing. Again, this makes good chemical sense because oxidation of carbohydrates using oxygen generates far more energy than can be obtained using other oxidants. Nonetheless, where oxygen is limited or absent, other electron donors are available for photosynthesis, and other electron acceptors can be used for respiration. The organisms that can use these alternative electron donors and electron acceptors are Bacteria and Archaea.
We’ve already noted that Bacteria and Archaea differ from Eukarya in cell structure, biochemistry, and genome organization, but it is the distinctive ways in which Bacteria and Archaea gather carbon and harvest energy that make them indispensable parts of nature. Prokaryotic organisms are thus the real foundation of the carbon cycle, capable of building and dismantling organic molecules throughout the full breadth of habitats on Earth.
26-7
26.2.1 Many photosynthetic bacteria do not produce oxygen.
In saline bays along the west coast of Australia and many other low-lying tropical coastlines, carpets of deep blue-green cover tidal flats along restricted lagoons (Fig. 26.7a). Called microbial mats, these carpets are densely packed communities of (mostly) bacteria and archaeons that thrive where animals and seaweeds cannot grow. The top surface of a microbial mat is dominated by cyanobacteria, bacteria that photosynthesize like plants and algae, using water as an electron donor and releasing oxygen gas (Fig. 26.7b). It is the photosynthetic pigments in the cyanobacteria that impart the blue-green color to microbial mats. The cyanobacteria (which synthesize their own organic molecules) and other, heterotrophic bacteria (which get their carbon from preformed organic molecules) respire aerobically, using O2 to oxidize organic molecules to CO2. Thus, at the mat surface, prokaryotic microorganisms carry out a biological carbon cycle much like that for plants and animals described in Chapter 25.
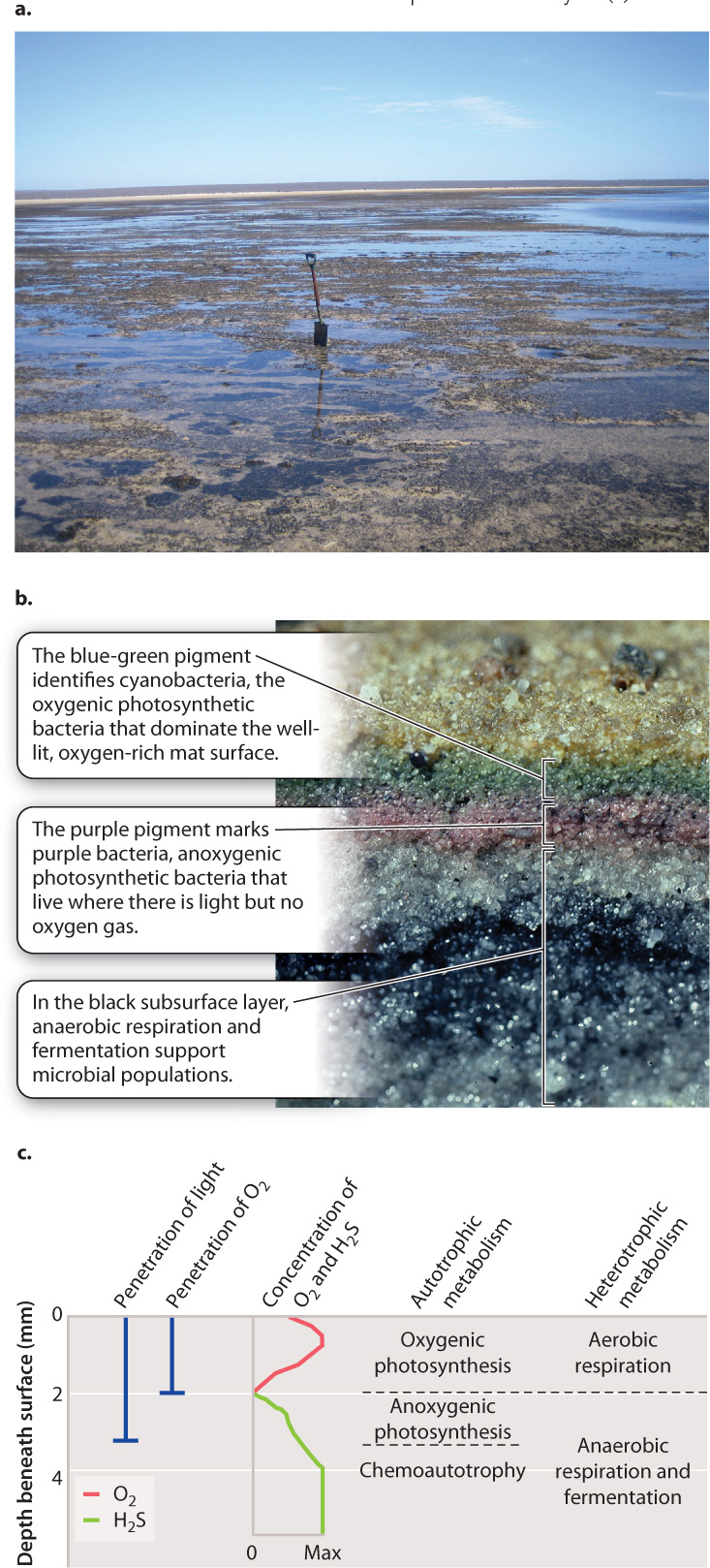
What happens beneath the mat surface is a different story. Mats generate a great deal of organic matter, and aerobic respiration of this material consumes O2 rapidly. In the mat interior below the level at which cyanobacteria supply oxygen during the day, O2 is supplied only slowly by diffusion, with the result that O2 becomes depleted within a few millimeters of the mat surface. Light is also greatly reduced beneath the mat surface, but in many mats, light actually penetrates a few millimeters deeper than oxygen (Fig. 26.7c). What organisms live where light is present and oxygen absent?
If we label CO2 with the radioisotope 14C, we find that carbon dioxide continues to be reduced to carbohydrates within this subsurface zone of the mat. In fact, the newly generated carbohydrates, identified by the 14C they have incorporated, occur in cells with bright purple or green pigments (Fig. 26.7b). These are anoxygenic photosynthetic bacteria, photosynthetic microorganisms that harvest light energy to drive the synthesis of carbohydrates, but which do not gain electrons from water and so do not generate oxygen gas in the process.
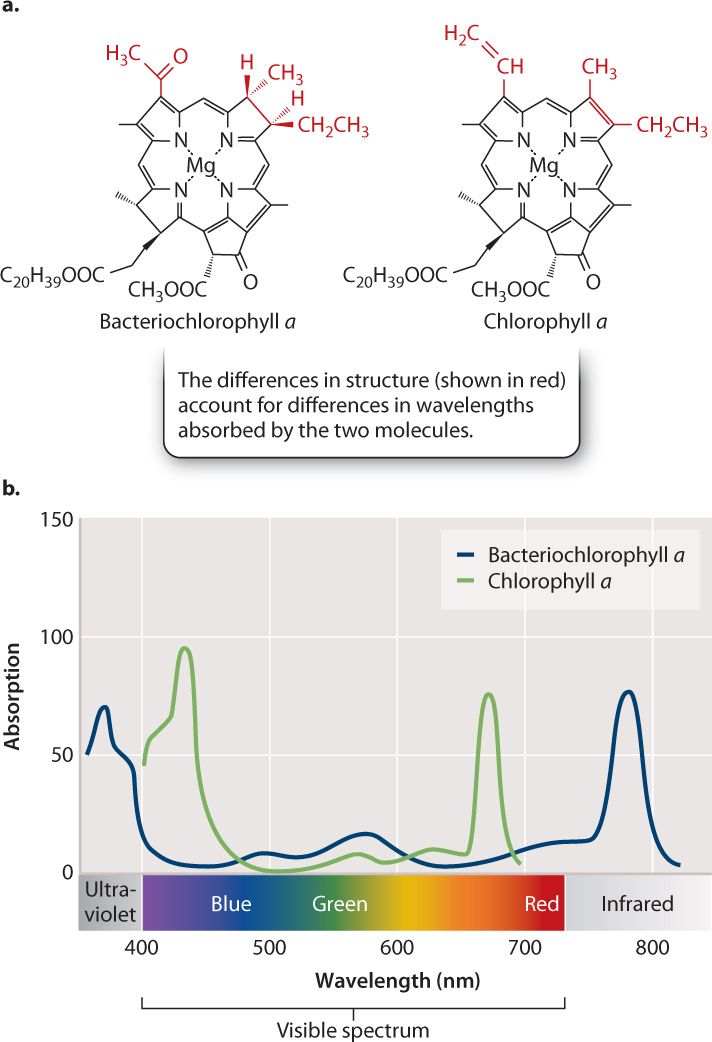
Anoxygenic photosynthetic bacteria absorb sunlight using bacteriochlorophyll, a light-harvesting pigment closely related to the chlorophyll found in plants, algae, and cyanobacteria. Fig. 26.8a shows the chemical structure of one form of bacteriochlorophyll, called bacteriochlorophyll a, and one form of chlorophyll, called chlorophyll a. Note the similarity in their overall structure. The two molecules absorb light of similar wavelengths as well, though small differences in their chemical structures account for small differences in which wavelengths they absorb more strongly (Fig. 26.8b).
Anoxygenic photosynthesis differs from oxygenic photosynthesis in another important way: It employs only a single photosystem. As discussed in Chapter 8, the amount of energy that can be captured this way is not sufficient to pull electrons from (that is, to oxidize) water and then use those electrons to reduce CO2, and so oxygenic photosynthesis has two photosystems in series. With only a single photosystem, bacteria that carry out anoxygenic photosynthesis use electron donors that donate their electrons more easily than water. As a result, these microorganisms do not release O2 as a by-product. Electron donors in anoxygenic photosynthesis include hydrogen sulfide (H2S), hydrogen gas (H2), ferrous iron (Fe2+), and even the arsenic compound arsenite (AsO33−). Where O2 is present, these compounds themselves become oxidized to other molecules and so are not available for photosynthesis. Therefore, microorganisms that conduct anoxygenic photosynthesis are restricted to sunlit habitats where O2 is absent.
26-8
26.2.2 Many bacteria respire without oxygen.
In microbial mat communities, heterotrophic microorganisms in the upper, oxygen-rich portion of the mat respire aerobically, just as we do, gaining carbon by taking in organic molecules from their surroundings and energy by using oxygen as an electron acceptor for the oxidation of those molecules. Does heterotrophy simply cease below the level where O2 disappears?
Not at all. Careful measurements show that organic molecules can be oxidized in the absence of O2. Oxygen is not unique in its capacity to accept electrons in redox reactions involving organic molecules, but where O2 is present it is used preferentially for respiration. This is because more energy is released by the oxidation of organic compounds by oxygen gas than by any alternative oxidant. In habitats where O2 is absent, oxidized forms of nitrogen (NO3−), sulfur (SO42−), manganese (Mn4+), iron (Fe3+), and even arsenic (AsO43−) are used as electron acceptors in cellular respiration.
Fermentation provides an alternative to cellular respiration as a way of extracting energy from organic molecules. Whereas cellular respiration is the full oxidation of carbon compounds to CO2, fermentation is the partial oxidation of carbon compounds to molecules that are less oxidized than CO2 (Chapter 7). Fermentation has one advantage over cellular respiration in that it does not require an external electron acceptor, such as O2. On the other hand, fermentation yields only a modest amount of energy.
Fermentation plays an important role in oxygen-poor environments that are rich in organic matter, such as landfills and the digestive tracts of animals. Here, the breakdown of organic molecules typically requires more than one organism, each able to metabolize different intermediates. These cooperating groups of fermenters enable the breakdown of substances that could not be metabolized by any one organism alone. Groups of fermenters thus form what is essentially an ecological solution to the metabolically difficult problem of gaining energy from complex organic molecules. Fermentation is widespread in Bacteria and Archaea, but except for yeasts, which are world-class fermenters, fermentation is of only minor importance in the energy metabolism of most Eukarya.
Therefore, where O2 is present, many different kinds of organisms participate in the carbon cycle. Photosynthetic plants, algae, and cyanobacteria all transform CO2 into organic molecules, and animals, fungi, single-celled eukaryotes, bacteria, and archaeons return CO2 to the environment by aerobic respiration. In Chapter 25, we noted that this biological carbon cycle leaks a bit. In other words, some of the organic carbon produced by photosynthesis escapes aerobic respiration and accumulates in oxygen-depleted waters or sediments. Only prokaryotic heterotrophs can complete the recycling of organic carbon in oxygen-poor environments. Thus, in carrying out anaerobic respiration or a diversity of fermentation reactions, prokaryotes play a major role in the carbon cycle. The key point is that prokaryotic organisms are required to sustain Earth’s carbon cycle, while eukaryotic organisms are optional.
26.2.3 Photoheterotrophs obtain energy from light but obtain carbon from preformed organic molecules.
In Chapter 6, we saw that organisms have two sources of energy: the sun (phototrophs) and chemical compounds (chemotrophs). Organisms also have two sources of the carbon needed for growth and other functions: inorganic molecules like CO2 (autotrophs) and organic molecules like glucose (heterotrophs). Taken together, this means that there are four ways that organisms acquire the energy and carbon they need (see Fig. 6.1).
26-9
Up to this point, we have considered only two of these ways. Plants, algae, and cyanobacteria are photoautotrophs, gaining energy from the sunlight and carbon from CO2, whereas animals, fungi, and many prokaryotes are chemoheterotrophs, gaining energy and carbon from organic molecules taken up from the environment. Among the Bacteria and Archaea, additional metabolisms are possible. Some microorganisms use the energy from sunlight to make ATP, just as plants do, but rather than reducing CO2 to make their own organic molecules, they rely on organic molecules obtained from the environment as the source of carbon for growth and other vital functions. These organisms are known as photoheterotrophs.
Photoheterotrophy can be advantageous in environments rich in dissolved organic compounds. It allows organisms to use all their absorbed light energy to make ATP while directing all absorbed organic molecules toward growth and reproduction. Examples of organisms capable of photoheterotrophy include heliobacteria and most green nonsulfur bacteria.
26.2.4 Chemoautotrophy is a uniquely prokaryotic metabolism.
On the deep seafloor, animals are relatively uncommon, their abundance limited by the slow descent of organic matter from surface oceans. Locally, however, where hydrothermal springs punctuate the deep seafloor, animal populations can be remarkably dense (Fig. 26.9). Why are animals so abundant around hydrothermal vents?

A clue comes from microbial mats. In mats where oxygen penetrates more deeply than light, CO2 continues to get reduced into organic molecules along the base of the zone containing oxygen. What is happening within the mat?
The organisms that incorporate CO2 into organic molecules deep within microbial mats and the organisms that provide food for abundant animals around deep-sea hydrothermal vents employ similar strategies for obtaining energy and carbon. Like plants and other autotrophs, they gain carbon by reducing CO2 to form carbohydrates. However, they obtain the energy to fuel this process not from sunlight but from chemical reactions. Hence, these microorganisms are called chemoautotrophs.
Our discussions of respiration emphasized that energy released by the oxidation of organic matter is used to generate ATP (Chapter 7). Chemoautotrophic microorganisms also use chemical reactions to generate ATP, but they use inorganic molecules present in their local environment. Chemoautotrophic prokaryotes use the oxidation of molecules such as H2, H2S, and even Fe2+ to generate the ATP and reducing power required to incorporate CO2 into organic molecules. In chemoautotrophic metabolism, the electron acceptor is most commonly O2 or nitrate (NO3−).
Because chemoautotrophy requires access to both oxidized and reduced molecules, chemoautotrophs tend to live along the interface between oxygen-rich and oxygen-poor environments. Microbial mats commonly have a sharp boundary between oxygen-rich and oxygen-poor layers, and at mid-ocean hydrothermal ridges, chemoautotophic prokaryotes (and the animals that harvest them) thrive where reduced gases released from Earth’s interior meet oxygen-rich seawater. The ability to use inorganic sources of chemical energy is widespread among Bacteria and Archaea, but entirely absent in Eukarya.
Question Quick Check 3
uFARPps7AK8fRYwaKfkMxn1jwJ3aakmneD0Bqn0h0uzZZL7aqUMPz6pUvHirbRHodNoLnM74Ga/k23gepkBjZrsdsrXtkiIetCP+XtVAuWP7ZYXWNMCVI5c66SmYVKw1rAl+GPjOQyHJKPE2PRqbhSBseugcCbKgxOMZ+SzkGfeZ+G5pEHMgtIsL1dXdX1oUmws3Hjf7XKs=26-10